Rickettsia conorii survival in THP-1 macrophages involves host lipid droplet alterations and active rickettsial protein production
Abstract
Rickettsia conorii is a Gram-negative, cytosolic intracellular bacterium that has classically been investigated in terms of endothelial cell infection. However, R. conorii and other human pathogenic Rickettsia species have evolved mechanisms to grow in various cell types, including macrophages, during mammalian infection. During infection of these phagocytes, R. conorii shifts the host cell's overall metabolism towards an anti-inflammatory M2 response, metabolically defined by an increase in host lipid metabolism and oxidative phosphorylation. Lipid metabolism has more recently been identified as a key regulator of host homeostasis through modulation of immune signalling and metabolism. Intracellular pathogens have adapted mechanisms of hijacking host metabolic pathways including host lipid catabolic pathways for various functions required for growth and survival. In the present study, we hypothesised that alterations of host lipid droplets initiated by lipid catabolic pathways during R. conorii infection is important for bacterial survival in macrophages. Herein, we determined that host lipid droplet modulation is initiated early during R. conorii infection, and these alterations rely on active bacteria and lipid catabolic pathways. We also find that these lipid catabolic pathways are essential for efficient bacterial survival. Unlike the mechanisms used by other intracellular pathogens, the catabolism of lipid droplets induced by R. conorii infection is independent of upstream host peroxisome proliferator-activated receptor-alpha (PPARα) signalling. Inhibition of PPARɣ signalling and lipid droplet accumulation in host cells cause a significant decrease in R. conorii survival suggesting a negative correlation with lipid droplet production and R. conorii survival. Together, these results strongly suggest that the modulation of lipid droplets in macrophage cells infected by R. conorii is an important and underappreciated aspect of the infection process.
Take Aways
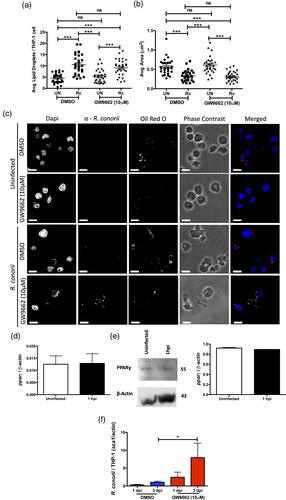
- Host lipid droplets are differentially altered in early and replicative stages of THP-1 macrophage infection with R. conorii.
- Lipid droplet alterations are initiated in a bacterial-dependent manner and do not require host peroxisome proliferator-activated receptors α or ɣ activation.
- Pharmacological inhibition of host lipid catabolic processes during R. conorii infection indicates a requirement of lipid catabolism for bacterial survival and initiation of lipid droplet modulation.
- A significant increase in host lipid droplets during infection has a negative impact on R. conorii survival in THP-1 macrophages.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


