Feminising Wolbachia disrupt Armadillidium vulgare insulin-like signalling pathway
Abstract
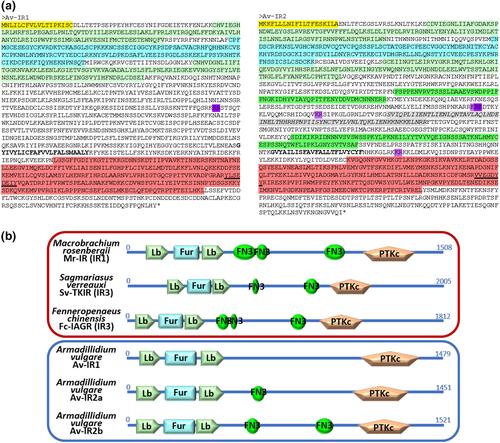
The endosymbiont Wolbachia feminises male isopods by making them refractory to the insulin-like masculinising hormone, which shunts the autocrine development of the androgenic glands. It was, therefore, proposed that Wolbachia silences the IR receptors, either by preventing their expression or by inactivating them. We describe here the two IR paralogs of Armadillidium vulgare. They displayed a conventional structure and belonged to a family widespread among isopods. Av-IR1 displayed an ubiquist expression, whereas the expression of Av-IR2 was restricted to the gonads. Both were constitutively expressed in males and females and throughout development. However, upon silencing, altered gland physiology and gene expression therein suggested antagonistic roles for Av-IR1 (androinhibiting) and Av-IR2 (androstimulating). They may function in tandem with regulating neurohormones, as a conditional platform that conveys insulin signalling. Wolbachia infection did not alter their expression patterns: leaving the IRs unscathed, the bacteria would suppress the secretion of the neurohormones, thus inducing body-wide IR deactivation and feminisation. Adult males injected with Wolbachia acquired an intersexed physiology. Their phenotypes and gene expressions mirrored the silencing of Av-IR1 only, suggesting that imperfect feminisation stems from a flawed invasion of the androstimulating centre, whereas in fully feminised males invasion would be complete in early juveniles.
Take Away
- Two antagonistic Insulin Receptors were characterised in Armadillidium vulgare.
- The IRs were involved in androstimulating and androinhibiting functions.
- Wolbachia-induced feminisation did not prevent the expression of the IRs.
- Imperfectly feminised intersexes phenocopied the silencing of Av-IR1 only.
- Wolbachia would deactivate the IRs by suppressing neurosecretory co-factors.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


