Fast and Reliable Synthesis of Melanin Nanoparticles with Fine-Tuned Metal Adsorption Capacities for Studying Heavy Metal Ions Uptake.
IF 2.4
Q2 NANOSCIENCE & NANOTECHNOLOGY
Nanotechnology, Science and Applications
Pub Date : 2021-05-24
eCollection Date: 2021-01-01
DOI:10.2147/NSA.S296722
引用次数: 5
Abstract
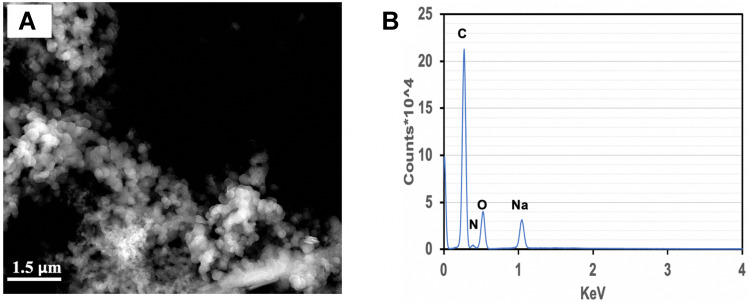
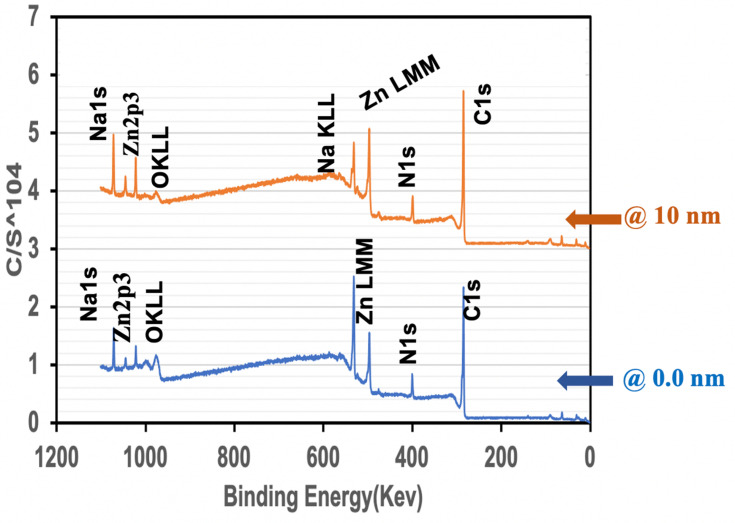
Purpose Adsorption and uptake of heavy metals by polymeric nanoparticles is driven by a variety of physicochemical processes. In this work, we examined heavy metal uptake by synthetic melanin nanoparticles and analyzed physicochemical properties that affect the extent of metal uptake by the nanoparticles. Methods Eumelanin nanoparticles were synthesized in a one-pot fast process from a 5,6-diacetoxy indole precursor that is hydrolyzed in situ into dihydroxy indole (DHI). The method allows the possibility of changing the level of sodium ions that ends up in the nanoparticles. Two variants of synthetic DHI–melanin (low-sodium and high sodium variants) were evaluated and demonstrated different relative adsorption efficiencies for heavy metal cations. Results and Discussion For the low-sodium DHI–melanin and in terms of percentages of metal ion removal, the relative order of extraction from 50 ppm solutions was Zn2+ > Cd2+ > Ni2+ > Co2+ > Cu2+ > Pb2+, with the extraction percentages ranging from 90% down to 76%, for a 30-minute adsorption time before equilibrium. The lower-sodium DHI–melanin consistently removed more Zn2+ than the higher-sodium variant. Electron microscopy (SEM) showed an increase in melanin particle size after metal ions uptake. In addition, X-ray photoelectron spectroscopy (XPS) of DHI–melanin particles with depth profiling after Zn ions uptake supported particle swelling and ion transport within the particles. Conclusion These initial studies showed the potential of this straightforward synthesis to obtain synthetic DHI–melanin nanoparticles similar to those from biological sources with the possibility to fine-tune their metal adsorption capacity. These synthetic nanoparticles can be used either for the removal of a variety of metal ions or to mimic and study mechanisms of metal uptake by melanin deriving from biological sources, with the potential to understand, for instance, differential heavy metal uptake by various melanic pigments.

快速可靠的合成具有微调金属吸附能力的黑色素纳米粒子用于研究重金属离子的吸收。
目的:高分子纳米颗粒对重金属的吸附和吸收是由多种物理化学过程驱动的。在这项工作中,我们研究了合成黑色素纳米颗粒对重金属的吸收,并分析了影响纳米颗粒对金属吸收程度的物理化学性质。方法:以5,6-二乙酰氧基吲哚前体原位水解为二羟基吲哚(DHI),采用一锅快速法制备真黑素纳米颗粒。这种方法可以改变纳米颗粒中钠离子的含量。对两种合成的dhi -黑色素(低钠型和高钠型)进行了评价,发现它们对重金属阳离子的相对吸附效率不同。结果与讨论:对于低钠的dhi -黑色素,从金属离子去除率的角度来看,在50 ppm溶液中,萃取的相对顺序为Zn2+ > Cd2+ > Ni2+ > Co2+ > Cu2+ > Pb2+,萃取率为90% ~ 76%,吸附时间为30 min,达到平衡。低钠dha -黑色素始终比高钠变体去除更多的Zn2+。电子显微镜(SEM)显示金属离子摄取后黑色素颗粒大小增加。此外,锌离子摄取后的dhi -黑色素粒子的x射线光电子能谱(XPS)支持粒子膨胀和离子在粒子内的传输。结论:这些初步的研究表明,这种简单的合成方法有可能获得与生物来源相似的合成dha -黑色素纳米颗粒,并有可能对其金属吸附能力进行微调。这些合成纳米颗粒既可用于去除各种金属离子,也可用于模拟和研究来自生物来源的黑色素对金属的吸收机制,例如,具有了解各种黑色色素对重金属的不同吸收的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nanotechnology, Science and Applications
NANOSCIENCE & NANOTECHNOLOGY-
CiteScore
11.70
自引率
0.00%
发文量
3
审稿时长
16 weeks
期刊介绍:
Nanotechnology, Science and Applications is an international, peer-reviewed, Open Access journal that focuses on the science of nanotechnology in a wide range of industrial and academic applications. The journal is characterized by the rapid reporting of reviews, original research, and application studies across all sectors, including engineering, optics, bio-medicine, cosmetics, textiles, resource sustainability and science. Applied research into nano-materials, particles, nano-structures and fabrication, diagnostics and analytics, drug delivery and toxicology constitute the primary direction of the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


