The neuronal ceroid lipofuscinosis-related protein CLN8 regulates endo-lysosomal dynamics and dendritic morphology
Abstract
Background Information
The endo-lysosomal system (ELS) comprises a set of membranous organelles responsible for transporting intracellular and extracellular components within cells. Defects in lysosomal proteins usually affect a large variety of processes and underlie many diseases, most of them with a strong neuronal impact. Mutations in the endoplasmic reticulum-resident CLN8 protein cause CLN8 disease. This condition is one of the 14 known neuronal ceroid lipofuscinoses (NCLs), a group of inherited diseases characterised by accumulation of lipofuscin-like pigments within lysosomes. Besides mediating the transport of soluble lysosomal proteins, recent research suggested a role for CLN8 in the transport of vesicles and lipids, and autophagy. However, the consequences of CLN8 deficiency on ELS structure and activity, as well as the potential impact on neuronal development, remain poorly characterised. Therefore, we performed CLN8 knockdown in neuronal and non-neuronal cell models to analyse structural, dynamic and functional changes in the ELS and to assess the impact of CLN8 deficiency on axodendritic development.
Results
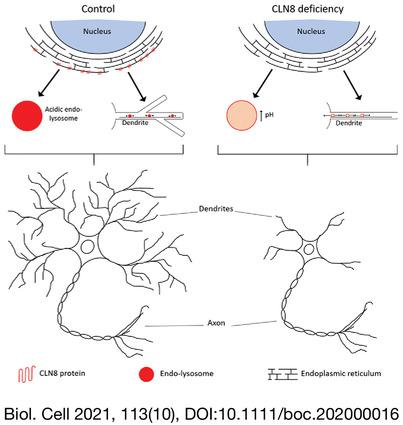
CLN8 knockdown increased the size of the Golgi apparatus, the number of mobile vesicles and the speed of endo-lysosomes. Using the fluorescent fusion protein mApple-LAMP1-pHluorin, we detected significant lysosomal alkalisation in CLN8-deficient cells. In turn, experiments in primary rat hippocampal neurons showed that CLN8 deficiency decreased the complexity and size of the somatodendritic compartment.
Conclusions
Our results suggest the participation of CLN8 in vesicular distribution, lysosomal pH and normal development of the dendritic tree. We speculate that the defects triggered by CLN8 deficiency on ELS structure and dynamics underlie morphological alterations in neurons, which ultimately lead to the characteristic neurodegeneration observed in this NCL.
Significance
This is, to our knowledge, the first characterisation of the effects of CLN8 dysfunction on the structure and dynamics of the ELS. Moreover, our findings suggest a novel role for CLN8 in somatodendritic development, which may account at least in part for the neuropathological manifestations associated with CLN8 disease.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


