Serum amyloid A3 deficiency impairs in vitro and in vivo adipocyte differentiation.
IF 3.5
4区 生物学
Q2 ENDOCRINOLOGY & METABOLISM
引用次数: 3
Abstract
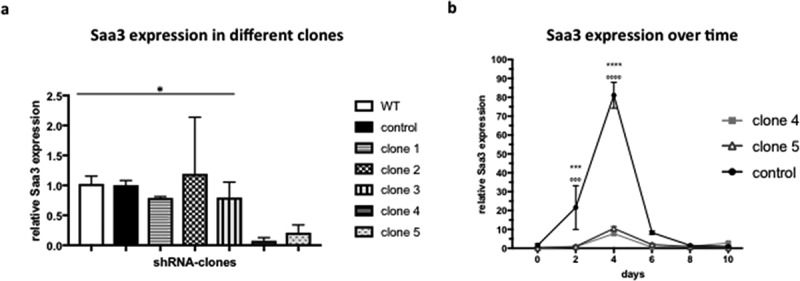
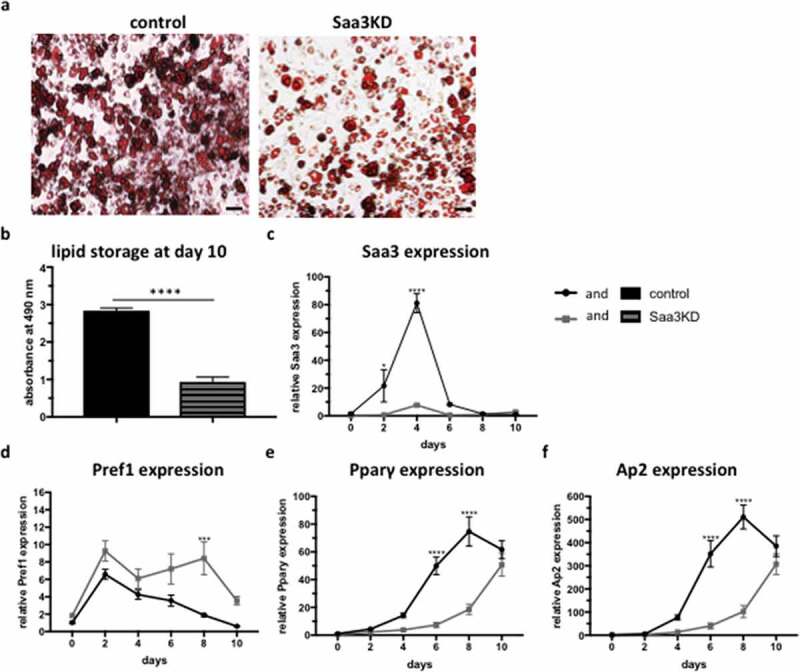
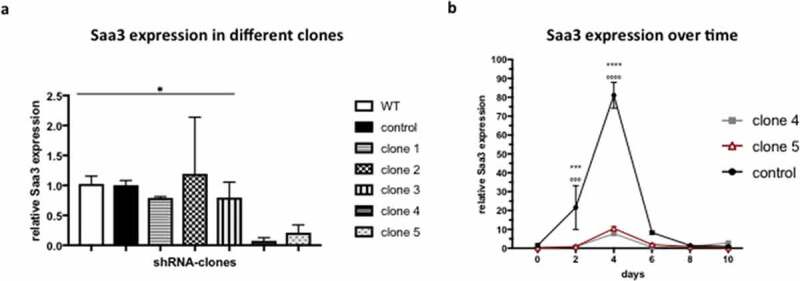
ABSTRACT Obesity, caused by an excess adipose tissue, is one of the biggest health-threats of the 21st century. Adipose tissue expansion occurs through two processes: (i) hypertrophy, and (ii) hyperplasia, the formation of new adipocytes, also termed adipogenesis. Recently, serum amyloid A3 (Saa3) has been implicated in adipogenesis. Therefore, the aim of this study was to investigate the effect of Saa3 on adipogenesis using both an in vitro and in vivo murine model. Saa3 gene silenced pre-adipocytes ha a lower expression of pro-adipogenic markers and less lipid accumulation, indicating impaired adipogenesis. Furthermore, male NUDE mice, injected with Saa3 gene silenced pre-adipocytes developed smaller fat pads with smaller adipocytes and lower expression of pro-adipogenic markers than their control counterparts. This confirms that Saa3 gene silencing indeed impairs adipogenesis, both in vitro and in vivo. These results indicate a clear role for Saa3 in adipogenesis and open new perspectives in the battle against obesity.
血清淀粉样蛋白A3缺乏损害体外和体内脂肪细胞分化。
肥胖是由过多的脂肪组织引起的,是21世纪最大的健康威胁之一。脂肪组织的扩张通过两个过程发生:(i)肥大,(ii)增生,即新脂肪细胞的形成,也称为脂肪生成。最近,血清淀粉样蛋白A3 (Saa3)与脂肪形成有关。因此,本研究的目的是通过体外和体内小鼠模型来研究Saa3对脂肪形成的影响。Saa3基因沉默的前脂肪细胞有较低的促脂肪标志物表达和较少的脂质积累,表明脂肪形成受损。此外,与对照组相比,注射Saa3基因沉默的前脂肪细胞的雄性裸鼠脂肪垫更小,脂肪细胞更小,促脂肪标志物的表达更低。这证实了Saa3基因沉默确实会在体外和体内损害脂肪形成。这些结果表明Saa3在脂肪形成中的明确作用,并为对抗肥胖开辟了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Adipocyte
Medicine-Histology
CiteScore
6.50
自引率
3.00%
发文量
46
审稿时长
32 weeks
期刊介绍:
Adipocyte recognizes that the adipose tissue is the largest endocrine organ in the body, and explores the link between dysfunctional adipose tissue and the growing number of chronic diseases including diabetes, hypertension, cardiovascular disease and cancer. Historically, the primary function of the adipose tissue was limited to energy storage and thermoregulation. However, a plethora of research over the past 3 decades has recognized the dynamic role of the adipose tissue and its contribution to a variety of physiological processes including reproduction, angiogenesis, apoptosis, inflammation, blood pressure, coagulation, fibrinolysis, immunity and general metabolic homeostasis. The field of Adipose Tissue research has grown tremendously, and Adipocyte is the first international peer-reviewed journal of its kind providing a multi-disciplinary forum for research focusing exclusively on all aspects of adipose tissue physiology and pathophysiology. Adipocyte accepts high-profile submissions in basic, translational and clinical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


