Disassembly of the apical junctional complex during the transmigration of Leptospira interrogans across polarized renal proximal tubule epithelial cells
IF 2.6
2区 生物学
Q3 CELL BIOLOGY
引用次数: 12
Abstract
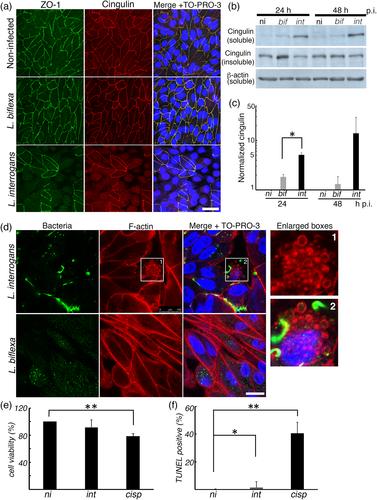
Bacterial pathogens have evolved multiple strategies to disassemble epithelial cell apical junctional complexes (AJCs) and infect epithelial cells. Leptospirosis is a widespread zoonotic infection, mainly caused by Leptospira interrogans, and its dissemination across host cell barriers is essential for its pathogenesis. However, the mechanism of bacterial dissemination across epithelial cell barriers remains poorly characterised. In this study, we analysed the interaction of L. interrogans with renal proximal tubule epithelial cells (RPTECs) and found that at 24 hr post‐infection, L. interrogans remain in close contact with the plasma membrane of the RPTEC by extracellularly adhering or crawling. Leptospira interrogans cleaved E‐cadherin and induced its endocytosis with release of the soluble N‐terminal fragment into the extracellular medium. Concomitantly, a gradual decrease in transepithelial electrical resistance (TEER), mislocalisation of AJC proteins (occludin, claudin‐10, ZO‐1, and cingulin) and cytoskeletal rearrangement were observed. Inhibition of clathrin‐mediated E‐cadherin endocytosis prevented the decrease in TEER. We showed that disassembly of AJCs in epithelial cells and transmigration of bacteria through the paracellular route are important for the dissemination of L. interrogans in the host.
钩端螺旋体在极化肾近端小管上皮细胞迁移过程中顶端连接复合体的解体
细菌病原体已经进化出多种策略来分解上皮细胞顶端连接复合物(AJCs)并感染上皮细胞。钩端螺旋体病是一种广泛的人畜共患感染,主要由钩端螺旋体引起,其传播跨越宿主细胞屏障是其发病的关键。然而,细菌传播跨越上皮细胞屏障的机制仍然不清楚。在这项研究中,我们分析了疑问乳杆菌与肾近端小管上皮细胞(RPTEC)的相互作用,发现在感染后24小时,疑问乳杆菌仍通过细胞外粘附或爬行与RPTEC的质膜密切接触。钩端螺旋体裂解e -钙粘蛋白并诱导其内吞,将可溶性n端片段释放到细胞外培养基中。同时,观察到经上皮电阻(TEER)逐渐下降,AJC蛋白(occludin, cludin -10, ZO-1和cingulin)错定位和细胞骨架重排。抑制网格蛋白介导的e -钙粘蛋白内吞作用可阻止TEER的降低。我们发现上皮细胞中AJCs的分解和细菌通过细胞旁途径的转运对于疑问乳杆菌在宿主中的传播是重要的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular Microbiology
生物-微生物学
CiteScore
9.70
自引率
0.00%
发文量
26
审稿时长
3 months
期刊介绍:
Cellular Microbiology aims to publish outstanding contributions to the understanding of interactions between microbes, prokaryotes and eukaryotes, and their host in the context of pathogenic or mutualistic relationships, including co-infections and microbiota. We welcome studies on single cells, animals and plants, and encourage the use of model hosts and organoid cultures. Submission on cell and molecular biological aspects of microbes, such as their intracellular organization or the establishment and maintenance of their architecture in relation to virulence and pathogenicity are also encouraged. Contributions must provide mechanistic insights supported by quantitative data obtained through imaging, cellular, biochemical, structural or genetic approaches.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


