Formulation, validation and evaluation studies on metaxalone and diclofenac potassium topical gel.
Environmental analysis, health and toxicology
Pub Date : 2021-03-01
Epub Date: 2021-01-11
DOI:10.5620/eaht.2021001
引用次数: 6
Abstract
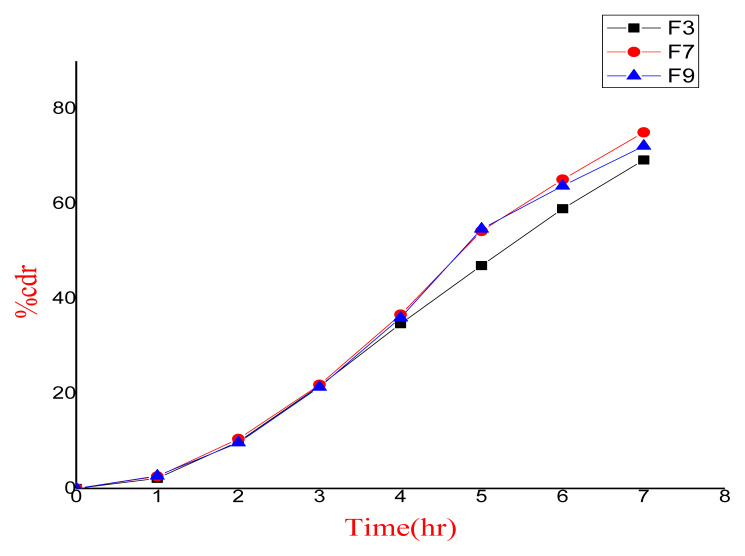
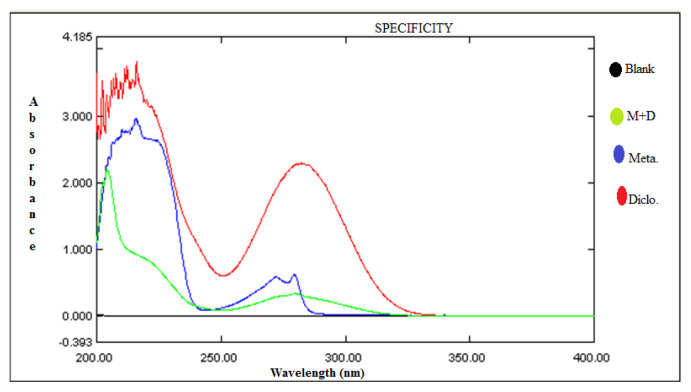
Twenty different batches of gels containing metaxalone and diclofenac potassium were prepared for topical application. These drugs act synergistically in the management of pain and inflammation. Gels were prepared by varying the type of gelling agent (ten batches each of hydroxyl propyl methyl cellulose and carbopol 934). The prepared gels were characterized and evaluated. Batch F7 emerged as the best batch on the basis of favourable pH, high drug content, homogeneity and drug release. HPLC (High-performance liquid chromatography) method validation of gel formulation was also carried out and the developed and validated method was found to be robust and accurate.
甲他alone双氯芬酸钾外用凝胶的处方、验证及评价研究。
制备了20个不同批次的甲他alone和双氯芬酸钾凝胶用于外用。这些药物在治疗疼痛和炎症方面起协同作用。通过不同类型的胶凝剂(羟丙基甲基纤维素和卡波波尔934各10批)制备凝胶。对制备的凝胶进行了表征和评价。F7批因pH适宜、药物含量高、均质性好、释放度高而被选为最佳批。对凝胶配方进行了高效液相色谱法验证,发现所建立的方法可靠、准确。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


