Modulating TRADD to restore cellular homeostasis and inhibit apoptosis
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 44
Abstract
Cell death in human diseases is often a consequence of disrupted cellular homeostasis. If cell death is prevented without restoring cellular homeostasis, it may lead to a persistent dysfunctional and pathological state. Although mechanisms of cell death have been thoroughly investigated1–3, it remains unclear how homeostasis can be restored after inhibition of cell death. Here we identify TRADD4–6, an adaptor protein, as a direct regulator of both cellular homeostasis and apoptosis. TRADD modulates cellular homeostasis by inhibiting K63-linked ubiquitination of beclin 1 mediated by TRAF2, cIAP1 and cIAP2, thereby reducing autophagy. TRADD deficiency inhibits RIPK1-dependent extrinsic apoptosis and proteasomal stress-induced intrinsic apoptosis. We also show that the small molecules ICCB-19 and Apt-1 bind to a pocket on the N-terminal TRAF2-binding domain of TRADD (TRADD-N), which interacts with the C-terminal domain (TRADD-C) and TRAF2 to modulate the ubiquitination of RIPK1 and beclin 1. Inhibition of TRADD by ICCB-19 or Apt-1 blocks apoptosis and restores cellular homeostasis by activating autophagy in cells with accumulated mutant tau, α-synuclein, or huntingtin. Treatment with Apt-1 restored proteostasis and inhibited cell death in a mouse model of proteinopathy induced by mutant tau(P301S). We conclude that pharmacological targeting of TRADD may represent a promising strategy for inhibiting cell death and restoring homeostasis to treat human diseases. The adaptor protein TRADD is a regulator of both cellular homeostasis and apoptosis, and represents a potential therapeutic target for human diseases.
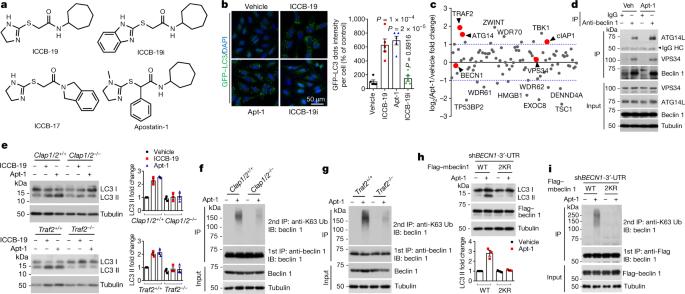
调节 TRADD 以恢复细胞平衡并抑制细胞凋亡
人类疾病中的细胞死亡往往是细胞平衡被破坏的结果。如果只阻止细胞死亡而不恢复细胞平衡,可能会导致持续的功能失调和病理状态。尽管细胞死亡的机制已被深入研究1-3,但抑制细胞死亡后如何恢复细胞稳态仍不清楚。在这里,我们发现适配蛋白 TRADD4-6 是细胞稳态和细胞凋亡的直接调节因子。TRADD 通过抑制 TRAF2、cIAP1 和 cIAP2 介导的 K63 链接泛素化 beclin 1 来调节细胞稳态,从而减少自噬。TRADD 缺乏会抑制 RIPK1 依赖性外源性凋亡和蛋白酶体应激诱导的内源性凋亡。我们还发现,小分子 ICCB-19 和 Apt-1 与 TRADD 的 N 端 TRAF2 结合结构域(TRADD-N)上的一个口袋结合,该口袋与 C 端结构域(TRADD-C)和 TRAF2 相互作用,从而调节 RIPK1 和 beclin 1 的泛素化。用ICCB-19或Apt-1抑制TRADD可阻止细胞凋亡,并通过激活细胞自噬恢复细胞平衡。在突变型 tau(P301S)诱导的蛋白病小鼠模型中,用 Apt-1 治疗可恢复蛋白稳态并抑制细胞死亡。我们的结论是,以 TRADD 为药理靶点可能是抑制细胞死亡和恢复平衡以治疗人类疾病的一种有前途的策略。适配蛋白TRADD是细胞稳态和细胞凋亡的调控因子,是人类疾病的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


