Defluorinative alkylation of 1-trifluoromethyl alkenes with alkyl radicals derived from visible light-induced deoxygenation of xanthate salts: synthesis of gem-difluoroalkenes†
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Catalyst- and metal-free difluoroallylation of alkyl precursors with trifluoromethyl alkenes for the synthesis of gem-difluoroalkenes is appealing and challenging. We herein describe a visible light-induced approach that enables deoxygenative difluoroallylation of abundant alcohols via xanthate salts with trifluoromethyl alkenes, where xanthate salts work as a photoreductant and an alkylating reagent, avoiding the use of external catalysts. This one-pot method can accommodate primary, secondary and tertiary alcohols with good functionality tolerance and be successfully applied to the late-stage functionalization of natural products and drugs.
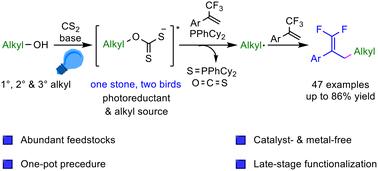
1-三氟甲基烯烃与黄原酸酯盐的可见光诱导脱氧衍生的烷基的脱氟烷基化:宝石二氟烯烃的合成†
烷基前体与三氟甲基烯烃的无催化剂和无金属二氟烯丙基化用于合成宝石二氟烯烃具有吸引力和挑战性。我们在此描述了一种可见光诱导的方法,该方法能够通过黄原酸酯盐与三氟甲基烯烃对丰富的醇进行脱氧二氟烯丙基化,其中黄原酸酯作为光还原剂和烷基化试剂,避免使用外部催化剂。这种一锅法可以容纳具有良好功能耐受性的伯醇、仲醇和叔醇,并成功应用于天然产物和药物的后期功能化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


