A biomimetic platelet based on assembling peptides initiates artificial coagulation
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 27
Abstract
Platelets play a critical role in the regulation of coagulation, one of the essential processes in life, attracting great attention. However, mimicking platelets for in vivo artificial coagulation is still a great challenge due to the complexity of the process. Here, we design platelet-like nanoparticles (pNPs) based on self-assembled peptides that initiate coagulation and form clots in blood vessels. The pNPs first bind specifically to a membrane glycoprotein (i.e., CD105) overexpressed on angiogenetic endothelial cells in the tumor site and simultaneously transform into activated platelet-like nanofibers (apNFs) through ligand-receptor interactions. Next, the apNFs expose more binding sites and recruit and activate additional pNPs, forming artificial clots in both phantom and animal models. The pNPs are proven to be safe in mice without systemic coagulation. The self-assembling peptides mimic platelets and achieve artificial coagulation in vivo, thus providing a promising therapeutic strategy for tumors.
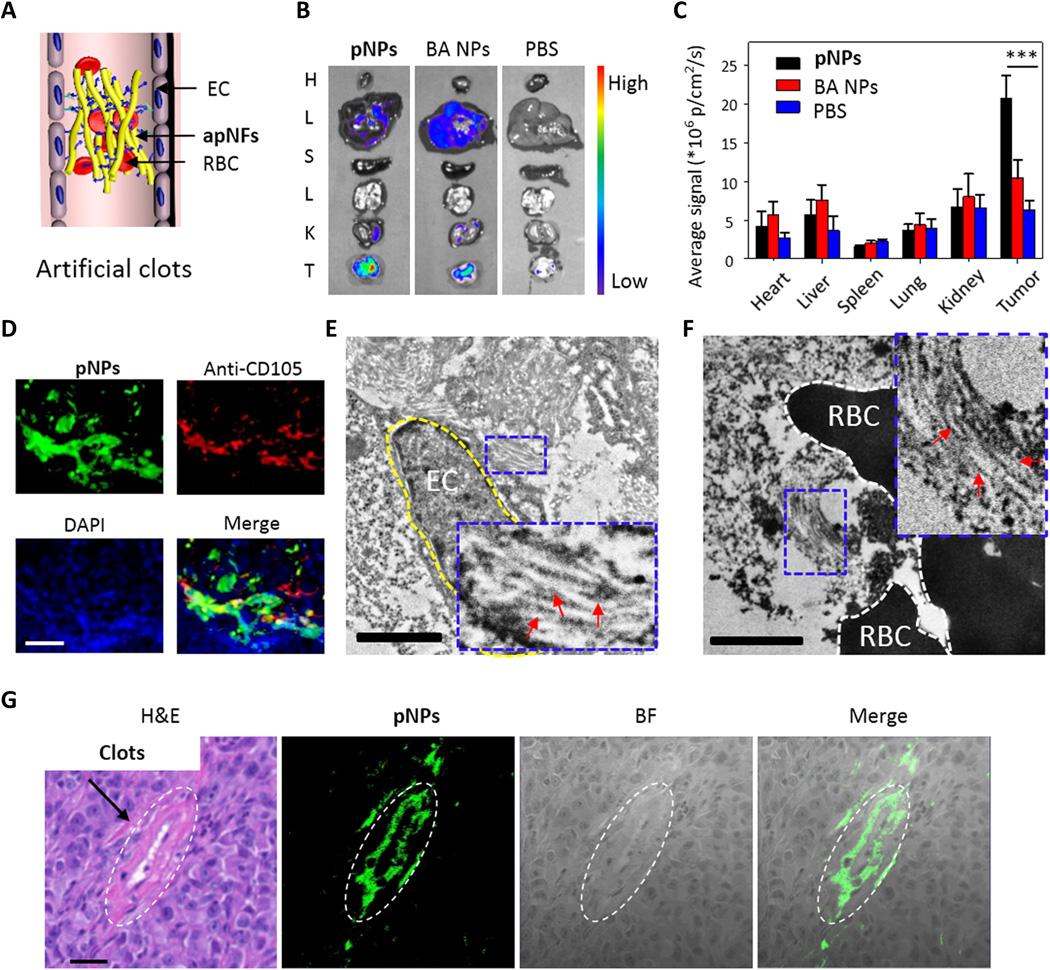
基于组装肽的仿生血小板启动人工凝血。
血小板在凝血调节中发挥着关键作用,凝血是生命中的重要过程之一,引起了人们的极大关注。然而,由于过程的复杂性,模拟血小板进行体内人工凝血仍然是一个巨大的挑战。在这里,我们设计了基于自组装肽的血小板样纳米颗粒(pNP),该肽可以启动凝血并在血管中形成凝块。pNP首先与肿瘤部位血管生成内皮细胞上过表达的膜糖蛋白(即CD105)特异性结合,同时通过配体-受体相互作用转化为活化的血小板样纳米纤维(apNFs)。接下来,apNF暴露出更多的结合位点,募集并激活额外的pNP,在体模和动物模型中形成人工血栓。pNP在没有全身凝血的小鼠中被证明是安全的。自组装肽模拟血小板并在体内实现人工凝血,从而为肿瘤的治疗提供了一种有前景的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


