Real-World Experience with Dolutegravir-Based Two-Drug Regimens.
IF 1.8
Q4 INFECTIOUS DISEASES
AIDS Research and Treatment
Pub Date : 2020-07-07
eCollection Date: 2020-01-01
DOI:10.1155/2020/5923256
引用次数: 6
Abstract
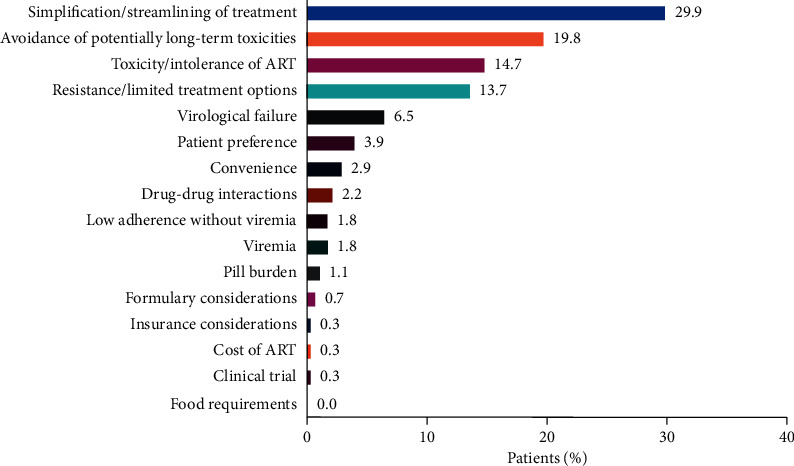
Background Dolutegravir-based 2-drug regimens (DTG 2DRs) are now accepted as alternatives to 3-drug regimens for HIV antiretroviral treatment (ART); however, literature on physician drivers for prescribing DTG 2DR is sparse. This study evaluated treatment patterns of DTG 2DR components in clinical practice in the US. Methods This was a retrospective chart review in adult patients in care in the US with HIV-1 who received DTG 2DR prior to July 31, 2017, with follow-up until January 30, 2018. Primary objectives of the study were to determine reasons for patients initiating DTG 2DR and to describe the demographics and clinical characteristics. All analyses were descriptive. Results Overall, 278 patients received DTG 2DR (male: 70%; mean age: 56 years). Most patients were treatment experienced (98%), with a mean 13.5 years of prior ART. DTG was most commonly paired with darunavir (55%) or rilpivirine (27%). The most common physician-reported reasons for initiating DTG 2DR were treatment simplification/streamlining (30%) and avoidance of potential long-term toxicities (20%). Before starting DTG 2DR, 42% of patients were virologically suppressed; of those, 95% maintained suppression while on DTG 2DR. Of the 50% of patients with detectable viral load before DTG 2DR, 79% achieved and maintained virologic suppression on DTG 2DR during follow-up. There were no virologic data for 8% of patients prior to starting DTG 2DR. Only 15 patients discontinued DTG 2DR, of whom 4 (27%) discontinued due to virologic failure. Conclusions Prior to commercial availability of the single-tablet 2DRs, DTG 2DR components were primarily used in treatment-experienced patients for treatment simplification and avoidance of long-term toxicities. Many of these patients achieved and maintained virologic suppression, with low discontinuation rates.
以dolutegravvir为基础的双药方案的实际经验。
背景:基于dolutegravvir的2药方案(DTG 2DRs)现在被接受为HIV抗逆转录病毒治疗(ART) 3药方案的替代方案;然而,关于医生驱动处方DTG 2DR的文献很少。本研究评估了美国临床实践中DTG 2DR成分的治疗模式。方法:这是对2017年7月31日前接受DTG 2DR治疗的美国HIV-1成年患者的回顾性图表回顾,随访至2018年1月30日。该研究的主要目的是确定患者启动DTG 2DR的原因,并描述人口统计学和临床特征。所有的分析都是描述性的。结果:总体而言,278例患者接受了DTG 2DR治疗(男性占70%;平均年龄:56岁)。大多数患者(98%)接受过治疗,平均接受过13.5年抗逆转录病毒治疗。DTG最常与darunavir(55%)或rilpivirine(27%)配对。医生报告的启动DTG 2DR的最常见原因是治疗简化/简化(30%)和避免潜在的长期毒性(20%)。在开始DTG 2DR之前,42%的患者得到病毒学抑制;其中95%的患者在服用DTG 2DR时仍保持抑制。在50%的患者在DTG 2DR前检测到病毒载量,79%的患者在随访期间达到并维持了DTG 2DR的病毒学抑制。在开始DTG 2DR之前,8%的患者没有病毒学数据。只有15例患者停止使用DTG 2DR,其中4例(27%)因病毒学失败而停止使用。结论:在单片2DR商业化之前,DTG 2DR成分主要用于治疗经验丰富的患者,以简化治疗并避免长期毒性。许多患者达到并维持病毒学抑制,停药率低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

AIDS Research and Treatment
INFECTIOUS DISEASES-
CiteScore
3.10
自引率
0.00%
发文量
13
审稿时长
18 weeks
期刊介绍:
AIDS Research and Treatment is a peer-reviewed, Open Access journal that publishes original research articles, review articles, and clinical studies focused on all aspects of HIV and AIDS, from the molecular basis of disease to translational and clinical research. In addition, articles relating to prevention, education, and behavior change will be considered
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


