The stair-step approach in treatment of anovulatory PCOS patients.
IF 1.8
Q1 OBSTETRICS & GYNECOLOGY
Therapeutic advances in reproductive health
Pub Date : 2020-05-22
eCollection Date: 2020-01-01
DOI:10.1177/2633494120908818
引用次数: 6
Abstract
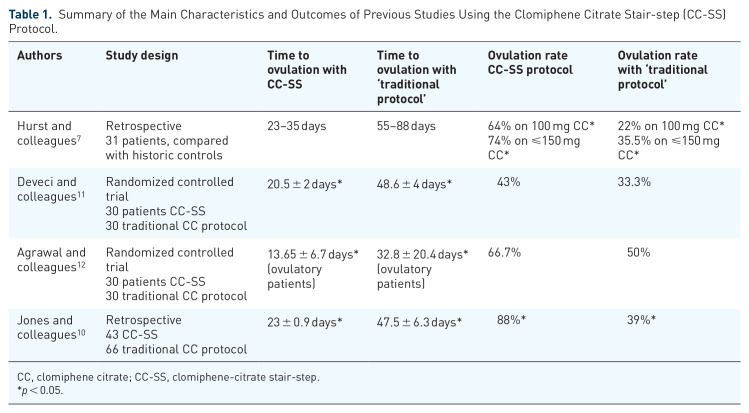
Clomiphene citrate (CC) is a widely accepted first-line treatment for anovulatory patients with polycystic ovarian syndrome (PCOS). The current practice is to prescribe CC with gradual dose increments until ovulation is achieved. Typically, progesterone withdrawal bleeding is induced between each dose increment and before the commencement of gonadotropin therapy in CC-resistant patients. It has been recently suggested that dose increments of CC can be administered once failure to induce ovulation at a certain dose has been documented, without induction of progesterone withdrawal bleeding, and this approach has been nicknamed the clomiphene-citrate stair-step (CC-SS) protocol. The same principle has been found feasible before introducing gonadotropin therapy in CC-resistant PCOS patients. Our objective was to review the world literature on the CC-SS protocol and to summarize our own experience with extending the CC-SS approach to initiation of gonadotropin therapy. Studies on CC-SS protocol (n = 4) have found that this approach leads to a significant reduction of the time to ovulation and to an increased ovulation rate. In our own retrospective case series, 18 CC-resistant PCOS patients initiated gonadotropin stimulation without induction of progesterone withdrawal bleeding, using the chronic low-dose regimen. The time to ovulation in the study group was 54.2 ± 6.2 days, while the estimated time to ovulation calculated according to the traditional approach was approximately 110 days. The clinical pregnancy rate was 44% (8/18), and all pregnancies were singletons. One patient miscarried; hence, the live birth rate was 38.9% (7/18). In summary, the CC-SS approach and its extension to the initiation of gonadotropin therapy results in considerable reduction of the time to ovulation, and favorable ovulation rates and reproductive outcome. Large-scale confirmation of these findings by properly designed randomized controlled trials may lead to a change of practice in the treatment of anovulatory infertility in PCOS patients, allowing simplification of treatment and a shorter time to ovulation and pregnancy.

阶梯式治疗无排卵性多囊卵巢综合征。
枸橼酸克罗米芬(CC)是一种被广泛接受的治疗无排卵多囊卵巢综合征(PCOS)的一线药物。目前的做法是开具CC,剂量逐渐增加,直到达到排卵。典型地,在每次剂量增加和开始促性腺激素治疗之前,在cc耐药患者中诱发黄体酮戒断性出血。最近有人建议,一旦证明在一定剂量下不能诱导排卵,就可以增加CC的剂量,而不会诱导黄体酮戒断性出血,这种方法被称为克罗米芬-柠檬酸盐阶梯(CC- ss)方案。在对cc抵抗性PCOS患者引入促性腺激素治疗之前,发现同样的原理是可行的。我们的目的是回顾世界上关于CC-SS方案的文献,并总结我们自己将CC-SS方法扩展到促性腺激素治疗开始的经验。CC-SS方案的研究(n = 4)发现,该方法可显著缩短排卵时间,提高排卵率。在我们自己的回顾性病例系列中,18例cc耐药PCOS患者采用慢性低剂量方案,在未诱导黄体酮戒断性出血的情况下开始促性腺激素刺激。研究组的排卵时间为54.2±6.2天,而按传统方法计算的预计排卵时间约为110天。临床妊娠率44%(8/18),均为单胎。1例流产;活产率为38.9%(7/18)。总之,CC-SS方法及其扩展到开始促性腺激素治疗的结果显着缩短排卵时间,良好的排卵率和生殖结果。通过适当设计的随机对照试验对这些发现进行大规模证实,可能会导致PCOS患者无排卵性不孕的治疗实践的改变,从而简化治疗并缩短排卵和妊娠时间。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


