Structure-based engineering of α-ketoglutarate dependent oxygenases in fungal meroterpenoid biosynthesis
Abstract
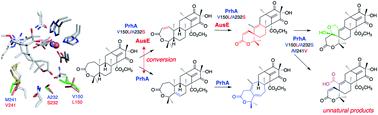
Non-heme iron- and α-ketoglutarate-dependent oxygenases (αKG OXs) are key enzymes that play a major role in diversifying the structure of fungal meroterpenoids. They activate a specific C–H bond of the substrate to first generate radical species, which is usually followed by oxygen rebound to produce cannonical hydroxylated products. However, in some cases remarkable chemistry induces dramatic structural changes in the molecular scaffolds, depending on the stereoelectronic characters of the substrate/intermediates and the resulting conformational changes/movements of the active site of the enzyme. Their molecular bases have been extensively investigated by crystallographic structural analyses and structure-based mutagenesis, which revealed intimate structural details of the enzyme reactions. This information facilitates the manipulation of the enzyme reactions to create unnatural, novel molecules for drug discovery. This review summarizes recent progress in the structure-based engineering of αKG OX enzymes, involved in the biosynthesis of polyketide-derived fungal meroterpenoids. The literature published from 2016 through February 2022 is reviewed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


