Status Presens of Antiviral Drugs And Strategies: Part I: DNA Viruses and Retroviruses.
Advances in antiviral drug design
Pub Date : 2007-01-01
Epub Date: 2007-09-02
DOI:10.1016/S1075-8593(06)05001-5
引用次数: 1
Abstract
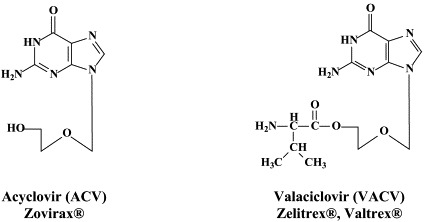
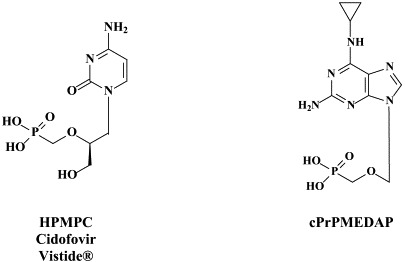
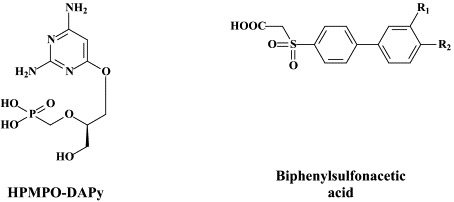
More than 40 compounds have been formally licensed for clinical use as antiviral drugs, and half of these are used for the treatment of HIV infections. The others have been approved for the therapy of herpesvirus (HSV, VZV, CMV), hepadnavirus (HBV), hepacivirus (HCV) and myxovirus (influenza, RSV) infections. New compounds are in clinical development or under preclinical evaluation, and, again, half of these are targeting HIV infections. Yet, quite a number of important viral pathogens (i.e. HPV, HCV, hemorrhagic fever viruses) remain in need of effective and/or improved antiviral therapies.
抗病毒药物和策略的现状:第一部分:DNA病毒和逆转录病毒。
超过40种化合物已被正式许可作为抗病毒药物用于临床,其中一半用于治疗艾滋病毒感染。其他药物已被批准用于治疗疱疹病毒(HSV、VZV、CMV)、肝炎病毒(HBV)、肝炎病毒(HCV)和黏液病毒(流感、RSV)感染。新的化合物正处于临床开发或临床前评估阶段,其中一半是针对艾滋病毒感染的。然而,相当多的重要病毒病原体(即人乳头瘤病毒、丙型肝炎病毒、出血热病毒)仍然需要有效和/或改进的抗病毒治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


