The entry of nanoparticles into solid tumours
IF 38.5
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 807
Abstract
The concept of nanoparticle transport through gaps between endothelial cells (inter-endothelial gaps) in the tumour blood vessel is a central paradigm in cancer nanomedicine. The size of these gaps was found to be up to 2,000 nm. This justified the development of nanoparticles to treat solid tumours as their size is small enough to extravasate and access the tumour microenvironment. Here we show that these inter-endothelial gaps are not responsible for the transport of nanoparticles into solid tumours. Instead, we found that up to 97% of nanoparticles enter tumours using an active process through endothelial cells. This result is derived from analysis of four different mouse models, three different types of human tumours, mathematical simulation and modelling, and two different types of imaging techniques. These results challenge our current rationale for developing cancer nanomedicine and suggest that understanding these active pathways will unlock strategies to enhance tumour accumulation. The dominant mechanism of nanoparticle entry into solid tumours has now been shown to be an active trans-endothelial pathway rather than the currently established passive transport via inter-endothelial gaps.
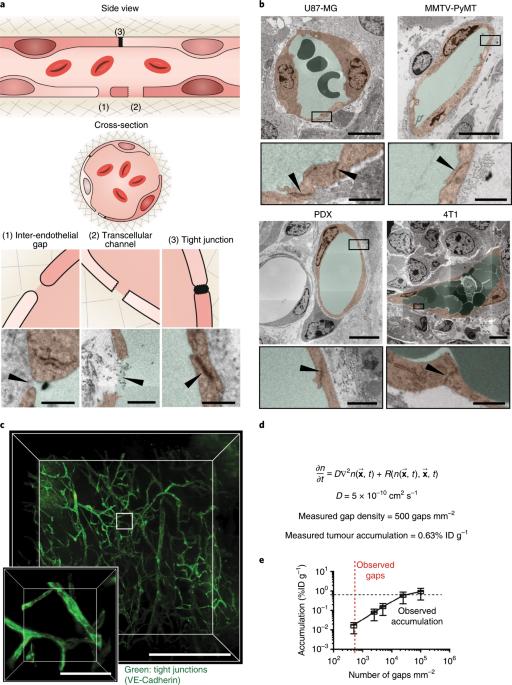
纳米粒子进入实体瘤
纳米粒子通过肿瘤血管内皮细胞之间的间隙(内皮间隙)运输的概念是癌症纳米医学的核心范式。研究发现,这些间隙的大小可达 2,000 纳米。这为开发治疗实体瘤的纳米颗粒提供了理由,因为它们的尺寸小到足以外渗并进入肿瘤微环境。在这里,我们发现这些内皮间隙并不是纳米粒子进入实体瘤的原因。相反,我们发现高达 97% 的纳米粒子是通过内皮细胞主动进入肿瘤的。这一结果是通过分析四种不同的小鼠模型、三种不同类型的人类肿瘤、数学模拟和建模以及两种不同类型的成像技术得出的。这些结果对我们目前开发癌症纳米药物的原理提出了挑战,并表明了解这些活性途径将开启增强肿瘤积累的策略。纳米粒子进入实体瘤的主要机制现已被证明是一种主动的跨内皮途径,而不是目前公认的通过内皮间隙的被动运输。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Materials
工程技术-材料科学:综合
CiteScore
62.20
自引率
0.70%
发文量
221
审稿时长
3.2 months
期刊介绍:
Nature Materials is a monthly multi-disciplinary journal aimed at bringing together cutting-edge research across the entire spectrum of materials science and engineering. It covers all applied and fundamental aspects of the synthesis/processing, structure/composition, properties, and performance of materials. The journal recognizes that materials research has an increasing impact on classical disciplines such as physics, chemistry, and biology.
Additionally, Nature Materials provides a forum for the development of a common identity among materials scientists and encourages interdisciplinary collaboration. It takes an integrated and balanced approach to all areas of materials research, fostering the exchange of ideas between scientists involved in different disciplines.
Nature Materials is an invaluable resource for scientists in academia and industry who are active in discovering and developing materials and materials-related concepts. It offers engaging and informative papers of exceptional significance and quality, with the aim of influencing the development of society in the future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


