Carbazole-fused coumarin based oxime esters (OXEs): efficient photoinitiators for sunlight driven free radical photopolymerization†
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The use of sunlight to initiate free radical polymerization under air is a key challenge. Due to the associated low light intensity, usual or commercial photoinitiators are characterized by a low efficiency. In this work, seventeen carbazole-fused coumarin-based oxime esters were developed as monocomponent and photocleavable (Type I) initiators of polymerization displaying excellent light absorption properties in the visible range. Compared to the benchmark photoinitiator (diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide), some of them (namely OXE-1 and OXE-5) could show photoinitiation performance similar to or better than the reference compound upon irradiation with a LED at 405 nm. The photoinitiation mechanism of these photoinitiators is proposed, supported by theoretical calculations; the detection of CO2 during photopolymerization was performed by means of Fourier transform infrared spectroscopy and the detection of radical species was performed by electron spin resonance analysis. Through direct laser writing, different objects exhibiting an excellent spatial resolution could be obtained. Parallel to the photoinitiating ability, the different photoinitiators also showed a thermal initiation behavior, meaning that these structures can serve both as thermal and photo-initiators on demand. Due to the high sensitivity of these structures to sunlight, OXE-1 and OXE-5 were also investigated as solar photoinitiators (reactive also in Central Europe during winter) and excellent monomer conversions could be obtained within one hour using a multifunctional acrylate monomer (Ebecryl 605). To the best of our knowledge, these two oxime esters constitute the first examples of sunlight activable oxime esters ever reported in the literature.
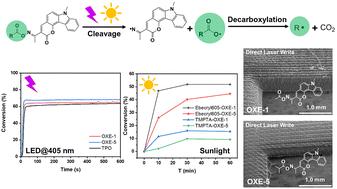
卡巴唑-香豆素基肟酯(OXEs):日光驱动自由基光聚合的高效光引发剂†
利用阳光在空气中引发自由基聚合是一个关键的挑战。由于相关的低光强,通常或商业光引发剂的特点是效率低。在这项工作中,开发了17种卡唑-香豆素基肟酯,作为单组分和光裂解(I型)聚合引发剂,在可见光范围内具有优异的光吸收性能。与基准光引发剂(二苯基(2,4,6-三甲基苯甲酰)氧化膦)相比,其中一些(即OXE-1和OXE-5)在405 nm的LED照射下可以表现出与参考化合物相似或更好的光引发性能。提出了这些光引发剂的光引发机理,并进行了理论计算;采用傅里叶变换红外光谱法检测光聚合过程中的CO2,采用电子自旋共振法检测自由基种类。通过激光直接书写,可以获得具有良好空间分辨率的不同物体。除了光引发能力外,不同的光引发剂还表现出热引发行为,这意味着这些结构可以根据需要同时作为热引发剂和光引发剂。由于这些结构对阳光的高度敏感性,OXE-1和OXE-5也被研究作为太阳光引发剂(在中欧冬季也有反应),使用多功能丙烯酸酯单体(Ebecryl 605)可以在一小时内获得优异的单体转化。据我们所知,这两种肟酯构成了文献中报道的阳光活化肟酯的第一个例子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


