Electrochemically driven α-thiocarbamylation via a dehydrocoupling strategy of β-ketoesters with amines and CS2†
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The development of atom- and step-economical methods for converting hazardous CS2 into harmless chemicals is a challenging endeavor. Herein, we disclose a feasible strategy for the electrochemical dehydrogenative coupling of CS2 and amines with β-ketoesters, which leads to dithiocarbamate intermediates in the presence of bases. In addition, inexpensive starting materials, broad substrate scope, and compatibility with natural product moieties make this efficient and sustainable reaction practical.
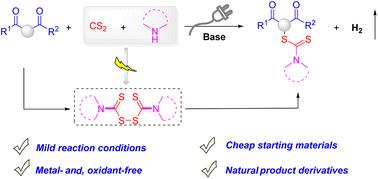
电化学驱动β-酮酯与胺和CS2†的脱氢偶联策略α-硫代氨基化
发展原子经济和阶梯经济的方法将有害的CS2转化为无害的化学品是一项具有挑战性的工作。在此,我们揭示了一种可行的策略,用于CS2和胺与β-酮酯的电化学脱氢偶联,从而在碱的存在下产生二硫代氨基甲酸酯中间体。此外,廉价的起始材料,广泛的底物范围,以及与天然产物部分的相容性使这种高效和可持续的反应成为现实。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


