2-Picolinic acid as a naturally occurring hydrogen bond donor for the preparation of cyclic carbonates from terminal/internal epoxides and CO2†
Abstract
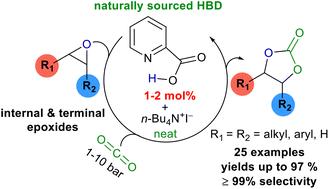
Naturally occurring 2-picolinic acid was uncovered as an off-the-shelf, non-toxic, commercially available, cost-effective and sustainable hydrogen bond donor (HBD) catalyst with a suitable halide co-catalyst for the cycloaddition of CO2 to both terminal and internal epoxides to prepare cyclic carbonates. The catalytic ability of the 2-picolinic acid/n-Bu4NI binary system was noticed when it was used to induce the insertion of CO2 into internal di-substituted epoxides as substrates. This is a rare instance of naturally sourced hydrogen bond donor catalyzed cycloaddition of CO2 to internal epoxides. Notably, 8 crucial internal di-substituted epoxides were converted to the corresponding cyclic carbonates with up to 97% yield and >99% selectivity with only 2 mol% catalyst loading. Additionally, 15 terminal mono-substituted epoxides were transformed under mild reaction conditions in the presence of CO2 (1 bar) to the related cyclic carbonates with up to 98% yield and >99% selectivity, with a low catalyst loading (1 mol%) and high turnover numbers (TON) and frequencies (TOF); TON/TOF (h−1) up to 97/5.4. The catalyst reusability experiment in which the reuse of 2-picolinic acid up to 5 times without significant loss of reactivity and a scale-up reaction with only 1 mol% catalyst loading was performed to highlight the practicality of this catalytic system. Density functional theory (DFT) calculations provided the reaction barriers for the different pyridine carboxylic acid catalysts employed in the title reaction and revealed that finding a suitable hydrogen bond donor catalyst hinges upon the interplay between the acidic strength and catalytic activity.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


