Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice.
Q1 Medicine
引用次数: 148
Abstract
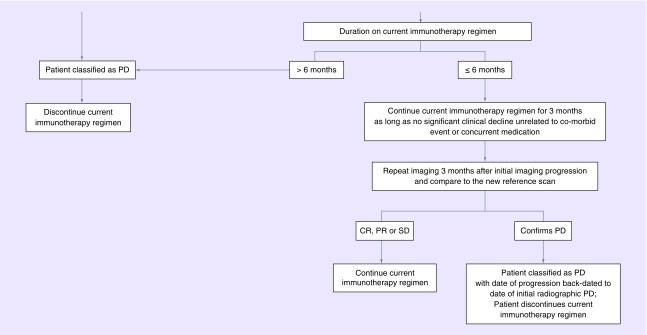
Between 2010 and 2014, the incidence rate of primary brain tumors in persons (aged >20 years) was 29.2 per 100,000; in children (aged <20 years), the rate was 5.81 per 100,000 [1]. Metastatic CNS tumors are known to be the most commonly occurring malignancy of the brain, although reporting for this disease is limited. Likely owing to multiple factors including improving survival from systemic malignancies, better tolerability of treatments, as well as timely and effective integration of supportive care, the incidence of CNS metastatic disease is expected to continue to increase [2]. Newer therapies and emphasis on clinical trial enrollment has made the need for effective approaches to assessing disease response even more critical. The Response Assessment in Neuro-Oncology (RANO) working group was established to improve the assessment of tumor response and selection of end points, specifically in the context of clinical trial [3]. There has been an evolution in determining which endpoints and criteria are most important in determining therapeutic response, specifically with advances in imaging modalities. In the era of computed tomography (CT), Levin et al. conducted a retrospective analysis of 100 brain tumor patients, in which they reviewed the predictive value of specific factors and its impact upon response to treatment. In this study, the combination of radionuclide and CT scans, as well as diligent monitoring of changes in dexamethasone dose were thought to be predictive of clinical deterioration and response to chemotherapy [4]. In the following decades, the field of neuro-oncology relied upon methods derived from the extracranial solid tumor oncology, notably the MacDonald criteria and the Response Evaluation Criteria in Solid Tumors (RECIST), both methods presenting shortcoming and challenges to effective response assessment in CNS tumors. In 1990, the MacDonald criteria were proposed as the standard for assessment of response and progression, specifically in patients with high-grade glioma. These criteria used the product of the maximal perpendicular diameters but also incorporated changes in corticosteroid doses as well as neurologic function [5]. In this scheme, adopting standards from medical oncology, four categories were recommended: complete response, in which there is disappearance of all enhancing disease concomitant with neurological improvement or stability AND absence of steroids, partial response or ≥50% reduction in enhancing disease as well as stable neurologic status and steroid use; progressive disease (PD) or ≥ 25% increase in enhancing disease or worsening neurologic status in the setting of stable or increasing steroid use and last, stable disease (SD) defined as all other scenarios [5]. RECIST was used occasionally for evaluation of treatment response in primary and metastatic brain tumors but most brain tumor trials used the MacDonald criteria preferentially, since it was felt that use of two orthogonal diameters (2D) may have advantages over measurement of a single, longest diameter (1D) for irregularly shaped brain tumors [5]. Among the challenges to use of earlier response criteria include lack of guidance on pseudoprogression, pseudoresponse and nonenhancing tumor progression. Historical challenges in the field have also been concerned around appropriate surrogates of response and endpoints [6]. RANO working groups was established to address some of these issues and provide guidance on assessment of response and endpoints in neuro-oncology clinical trials. Although the work of RANO initially focused on gliomas, its work has extended to many other areas of neuro-
神经肿瘤反应评估(RANO)标准在临床试验和临床实践中的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


