Nuclear cGAS suppresses DNA repair and promotes tumorigenesis
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 367
Abstract
Accurate repair of DNA double-stranded breaks by homologous recombination preserves genome integrity and inhibits tumorigenesis. Cyclic GMP–AMP synthase (cGAS) is a cytosolic DNA sensor that activates innate immunity by initiating the STING–IRF3–type I IFN signalling cascade1,2. Recognition of ruptured micronuclei by cGAS links genome instability to the innate immune response3,4, but the potential involvement of cGAS in DNA repair remains unknown. Here we demonstrate that cGAS inhibits homologous recombination in mouse and human models. DNA damage induces nuclear translocation of cGAS in a manner that is dependent on importin-α, and the phosphorylation of cGAS at tyrosine 215—mediated by B-lymphoid tyrosine kinase—facilitates the cytosolic retention of cGAS. In the nucleus, cGAS is recruited to double-stranded breaks and interacts with PARP1 via poly(ADP-ribose). The cGAS–PARP1 interaction impedes the formation of the PARP1–Timeless complex, and thereby suppresses homologous recombination. We show that knockdown of cGAS suppresses DNA damage and inhibits tumour growth both in vitro and in vivo. We conclude that nuclear cGAS suppresses homologous-recombination-mediated repair and promotes tumour growth, and that cGAS therefore represents a potential target for cancer prevention and therapy. DNA damage induces translocation of cyclic GMP–AMP synthase to the nucleus, where it suppresses homologous recombination by interfering with the formation of the PARP1–Timeless complex.
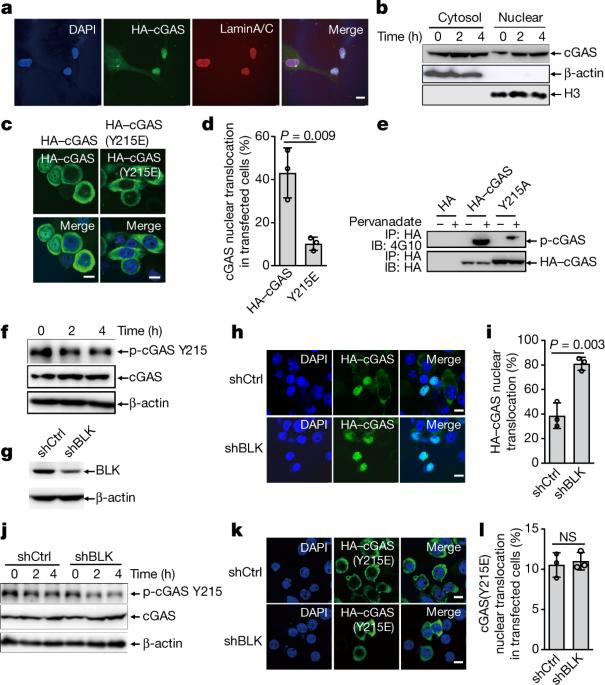
核 cGAS 抑制 DNA 修复并促进肿瘤发生
通过同源重组准确修复 DNA 双链断裂可保护基因组完整性并抑制肿瘤发生。环GMP-AMP合成酶(cGAS)是一种细胞膜DNA传感器,可通过启动STING-IRF3-I型IFN信号级联激活先天性免疫1,2。cGAS 识别破裂的微核将基因组不稳定性与先天性免疫反应联系起来3,4,但 cGAS 在 DNA 修复中的潜在参与仍然未知。在这里,我们证明了 cGAS 在小鼠和人类模型中抑制同源重组。DNA 损伤诱导 cGAS 核转位,这种转位依赖于导入素-α,而 B 淋巴细胞酪氨酸激酶介导的 cGAS 在酪氨酸 215 处的磷酸化促进了 cGAS 的胞浆保留。在细胞核中,cGAS 被招募到双链断裂处,并通过聚(ADP-核糖)与 PARP1 相互作用。cGAS 与 PARP1 的相互作用阻碍了 PARP1-Timeless 复合物的形成,从而抑制了同源重组。我们的研究表明,在体外和体内敲除 cGAS 可抑制 DNA 损伤并抑制肿瘤生长。我们的结论是,核cGAS抑制同源重组介导的修复,促进肿瘤生长,因此cGAS是癌症预防和治疗的潜在靶点。DNA 损伤诱导环 GMP-AMP 合成酶转位到细胞核,并在细胞核中通过干扰 PARP1-Timeless 复合物的形成来抑制同源重组。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


