Structural basis for regulation of human acetyl-CoA carboxylase
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 119
Abstract
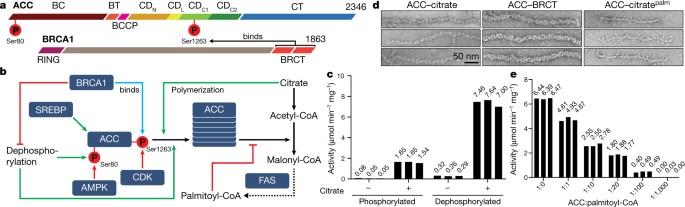
Acetyl-CoA carboxylase catalyses the ATP-dependent carboxylation of acetyl-CoA, a rate-limiting step in fatty acid biosynthesis1,2. Eukaryotic acetyl-CoA carboxylases are large, homodimeric multienzymes. Human acetyl-CoA carboxylase occurs in two isoforms: the metabolic, cytosolic ACC1, and ACC2, which is anchored to the outer mitochondrial membrane and controls fatty acid β-oxidation1,3. ACC1 is regulated by a complex interplay of phosphorylation, binding of allosteric regulators and protein–protein interactions, which is further linked to filament formation1,4–8. These filaments were discovered in vitro and in vivo 50 years ago7,9,10, but the structural basis of ACC1 polymerization and regulation remains unknown. Here, we identify distinct activated and inhibited ACC1 filament forms. We obtained cryo-electron microscopy structures of an activated filament that is allosterically induced by citrate (ACC–citrate), and an inactivated filament form that results from binding of the BRCT domains of the breast cancer type 1 susceptibility protein (BRCA1). While non-polymeric ACC1 is highly dynamic, filament formation locks ACC1 into different catalytically competent or incompetent conformational states. This unique mechanism of enzyme regulation via large-scale conformational changes observed in ACC1 has potential uses in engineering of switchable biosynthetic systems. Dissecting the regulation of acetyl-CoA carboxylase opens new paths towards counteracting upregulation of fatty acid biosynthesis in disease. Cryo-electron microscopy studies of distinct, catalytically active and inactive filaments of human acetyl-CoA carboxylase 1 reveal the structural basis of its regulation.
人类乙酰-CoA羧化酶调控的结构基础
乙酰-CoA羧化酶催化乙酰-CoA 的 ATP 依赖性羧化,这是脂肪酸生物合成的限速步骤1,2。真核生物乙酰-CoA羧化酶是大型的同源二聚体多酶。人类乙酰-CoA羧化酶有两种同工酶:一种是代谢型、细胞质型 ACC1,另一种是锚定在线粒体外膜上、控制脂肪酸 β 氧化的 ACC21,3。ACC1 受磷酸化、异位调节剂结合和蛋白质相互作用等复杂相互作用的调控,而这些相互作用又与丝的形成有关1,4-8。50 年前,人们在体外和体内发现了这些细丝7,9,10,但 ACC1 聚合和调控的结构基础仍不清楚。在这里,我们确定了不同的激活和抑制 ACC1 细丝形式。我们获得了由柠檬酸盐(ACC-柠檬酸盐)异构诱导的活化丝状结构,以及由乳腺癌 1 型易感蛋白(BRCA1)的 BRCT 结构域结合导致的失活丝状结构。非聚合 ACC1 具有很强的动态性,而丝状结构的形成则将 ACC1 锁定在不同的有催化能力或无催化能力的构象状态。在 ACC1 中观察到的这种通过大规模构象变化进行酶调控的独特机制在可切换生物合成系统工程中具有潜在用途。剖析乙酰-CoA羧化酶的调控为对抗疾病中脂肪酸生物合成的上调开辟了新的道路。对人类乙酰-CoA羧化酶 1 不同的催化活性和非活性丝的冷冻电镜研究揭示了其调控的结构基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


