KLHL22 activates amino-acid-dependent mTORC1 signalling to promote tumorigenesis and ageing
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 78
Abstract
The mechanistic target of rapamycin complex 1 (mTORC1) is a master regulator of cell growth that responds to a diverse set of environmental cues, including amino acids1,2. Deregulation of mTORC1 has been linked with metabolic diseases, cancer and ageing2–4. In response to amino acids, mTORC1 is recruited by the Rag GTPases to the lysosome, its site of activation5,6. The GATOR1 complex, consisting of DEPDC5, NPRL3 and NPRL2, displays GAP activity to inactivate Rag GTPases under amino-acid-deficient conditions 7 . However, it is unclear how the inhibitory function of GATOR1 is released upon amino acid stimulation. Here we find that in response to amino acids, the CUL3–KLHL22 E3 ubiquitin ligase promotes K48-linked polyubiquitination and degradation of DEPDC5, an essential subunit of GATOR1. KLHL22 plays a conserved role to mediate the activation of mTORC1 and downstream events in mammals and nematodes. Depletion of MEL-26, the Caenorhabditis elegans orthologue of KLHL22, extends worm lifespan. Moreover, KLHL22 levels are elevated in tumours of breast cancer patients, whereas DEPDC5 levels are correspondingly reduced. Depletion of KLHL22 in breast cancer cells suppresses tumour growth in nude mice. Therefore, pharmacological interventions targeting KLHL22 may have therapeutic potential for the treatment of breast cancer and age-related diseases. In response to amino acid stimulation, the ubiquitin E3 ligase CUL3–KLHL22 promotes the activation of mTORC1, which may drive tumour growth in breast cancer.
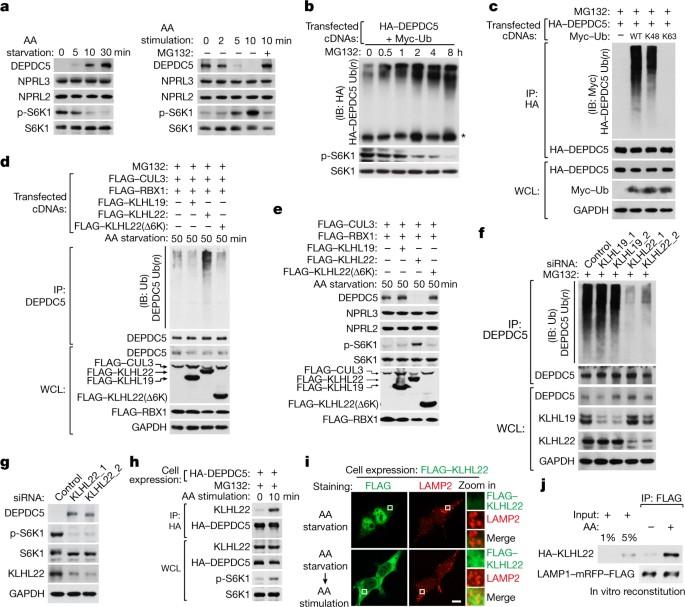
KLHL22 激活氨基酸依赖性 mTORC1 信号,促进肿瘤发生和衰老
雷帕霉素机理靶点复合体 1(mTORC1)是细胞生长的主调节因子,可对包括氨基酸在内的多种环境线索做出反应1,2。mTORC1 的失调与代谢性疾病、癌症和老化有关2-4。在氨基酸的作用下,mTORC1 被 Rag GTP 酶招募到溶酶体,即其激活部位5,6。由 DEPDC5、NPRL3 和 NPRL2 组成的 GATOR1 复合物具有 GAP 活性,能在氨基酸缺乏的条件下使 Rag GTPases 失活7 。然而,目前还不清楚 GATOR1 的抑制功能在氨基酸刺激下是如何释放的 。在这里,我们发现在氨基酸的作用下,CUL3-KLHL22 E3 泛素连接酶促进了与 K48 连接的多泛素化和 GATOR1 的一个重要亚基 DEPDC5 的降解。在哺乳动物和线虫中,KLHL22 在介导 mTORC1 激活和下游事件方面发挥着保守的作用。消耗草履虫 KLHL22 的直向同源物 MEL-26 可延长线虫的寿命。此外,乳腺癌患者肿瘤中的 KLHL22 水平升高,而 DEPDC5 水平则相应降低。消耗乳腺癌细胞中的 KLHL22 能抑制肿瘤在裸鼠体内的生长。因此,针对 KLHL22 的药物干预可能具有治疗乳腺癌和老年相关疾病的潜力。在氨基酸刺激下,泛素 E3 连接酶 CUL3-KLHL22 促进 mTORC1 的激活,这可能会推动乳腺癌中肿瘤的生长。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


