Palmitoylethanolamide in the Treatment of Failed Back Surgery Syndrome.
Q2 Medicine
引用次数: 16
Abstract
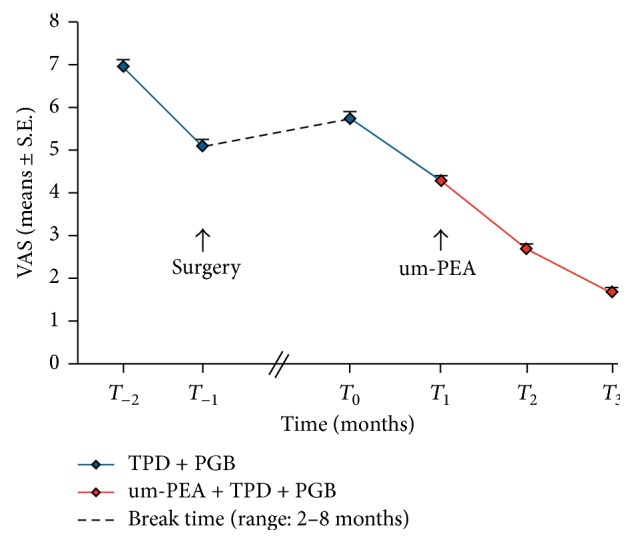
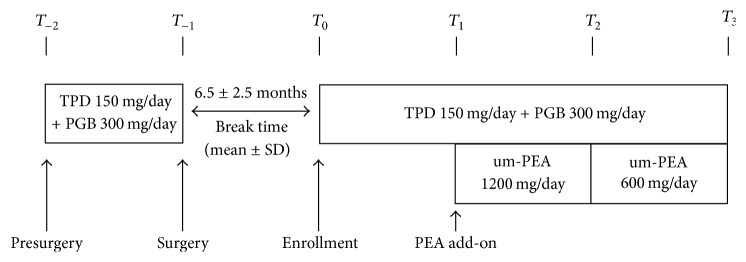
Introduction This observational study was designed to evaluate the efficacy of ultramicronized palmitoylethanolamide (um-PEA) (Normast®) administration, as add-on therapy for chronic pain, in the management of pain-resistant patients affected by failed back surgery syndrome. Methods A total of 35 patients were treated with tapentadol (TPD) and pregabalin (PGB). One month after the start of standard treatment, um-PEA was added for the next two months. Pain was evaluated by the Visual Analogue Scale (VAS) at the time of enrollment (T0) and after one (T1), two (T2), and three (T3) months. Results After the first month with TDP + PGB treatment only, VAS score decreased significantly from 5.7 ± 0.12 at the time of enrollment (T0) to 4.3 ± 0.11 (T1) (p < 0.0001); however, it failed to provide significant subjective improvement in pain symptoms. Addition of um-PEA led to a further and significant decrease in pain intensity, reaching VAS scores of 2.7 ± 0.09 (T2) and 1.7 ± 0.11 (T3, end of treatment) (p < 0.0001) without showing any side effects. Conclusions This observational study provides evidence, albeit preliminary, for the efficacy and safety of um-PEA (Normast) as part of a multimodal therapeutic regimen in the treatment of pain-resistant patients suffering from failed back surgery syndrome.
棕榈酰乙醇酰胺治疗背部手术失败综合征。
本观察性研究旨在评估超微化棕榈酰乙醇酰胺(um-PEA) (Normast®)作为慢性疼痛的附加治疗,在治疗背部手术失败综合征的疼痛耐受性患者中的疗效。方法:35例患者采用他他多(TPD)联合普瑞巴林(PGB)治疗。标准治疗开始一个月后,在接下来的两个月里添加um-PEA。在入组时(T0)和1个月(T1)、2个月(T2)和3个月(T3)后,采用视觉模拟评分(VAS)评估疼痛。结果:仅TDP + PGB治疗1个月后,VAS评分由入组时的5.7±0.12 (T0)降至4.3±0.11 (T1) (p < 0.0001);然而,它不能提供明显的主观改善疼痛症状。添加um-PEA可进一步显著降低疼痛强度,VAS评分分别为2.7±0.09 (T2)和1.7±0.11 (T3,治疗结束)(p < 0.0001),无任何副作用。结论:这项观察性研究为um-PEA (Normast)作为多模式治疗方案的一部分用于治疗背部手术失败综合征的疼痛耐受性患者的有效性和安全性提供了初步证据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Pain Research and Treatment
Medicine-Anesthesiology and Pain Medicine
CiteScore
3.60
自引率
0.00%
发文量
0
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


