GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates
IF 50
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 416
Abstract
GDF15 has potent anti-obesity effects, but its receptor was unknown. GFRAL has now been identified as the receptor and mediates GDF15''s effects through central actions in the hindbrain. Growth differentiation factor 15 (GDF15), a distant member of the transforming growth factor (TGF)-β family, is a secreted protein that circulates as a 25-kDa dimer. In humans, elevated GDF15 correlates with weight loss, and the administration of GDF15 to mice with obesity reduces body weight, at least in part, by decreasing food intake. The mechanisms through which GDF15 reduces body weight remain poorly understood, because the cognate receptor for GDF15 is unknown. Here we show that recombinant GDF15 induces weight loss in mice fed a high-fat diet and in nonhuman primates with spontaneous obesity. Furthermore, we find that GDF15 binds with high affinity to GDNF family receptor α–like (GFRAL), a distant relative of receptors for a distinct class of the TGF-β superfamily ligands. Gfral is expressed in neurons of the area postrema and nucleus of the solitary tract in mice and humans, and genetic deletion of the receptor abrogates the ability of GDF15 to decrease food intake and body weight in mice. In addition, diet-induced obesity and insulin resistance are exacerbated in GFRAL-deficient mice, suggesting a homeostatic role for this receptor in metabolism. Finally, we demonstrate that GDF15-induced cell signaling requires the interaction of GFRAL with the coreceptor RET. Our data identify GFRAL as a new regulator of body weight and as the bona fide receptor mediating the metabolic effects of GDF15, enabling a more comprehensive assessment of GDF15 as a potential pharmacotherapy for the treatment of obesity.
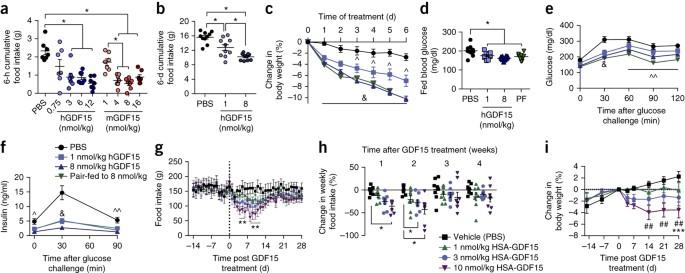
GFRAL 是 GDF15 的受体,配体可促进小鼠和非人灵长类动物的体重减轻
GDF15 具有强大的抗肥胖作用,但其受体却不为人知。现在,GFRAL 已被确认为受体,并通过后脑的中枢作用介导 GDF15 的效应。生长分化因子 15(GDF15)是转化生长因子(TGF)-β 家族的远亲,是一种分泌蛋白,以 25 kDa 的二聚体形式循环。在人体中,GDF15 的升高与体重减轻有关,给肥胖症小鼠注射 GDF15 至少部分是通过减少食物摄入量来减轻体重的。由于 GDF15 的同源受体未知,人们对 GDF15 减轻体重的机制仍然知之甚少。在这里,我们发现重组 GDF15 能诱导高脂饮食小鼠和自发性肥胖的非人灵长类动物减轻体重。此外,我们还发现 GDF15 与 GDNF 家族受体 α-样(GFRAL)具有高亲和力,后者是 TGF-β 超家族配体中一类独特受体的远亲。Gfral 在小鼠和人的后叶区和孤束核的神经元中表达,受体的基因缺失会削弱 GDF15 减少小鼠食物摄入量和体重的能力。此外,饮食引起的肥胖和胰岛素抵抗在 GFRAL 缺失的小鼠中会加剧,这表明该受体在新陈代谢中起着平衡作用。最后,我们证明 GDF15 诱导的细胞信号传导需要 GFRAL 与核心受体 RET 的相互作用。我们的数据确定了 GFRAL 是体重的新调节因子,也是介导 GDF15 代谢效应的真正受体,从而能够对 GDF15 作为治疗肥胖症的潜在药物疗法进行更全面的评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


