Structural analysis of BRCA1 reveals modification hotspot
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 15
Abstract
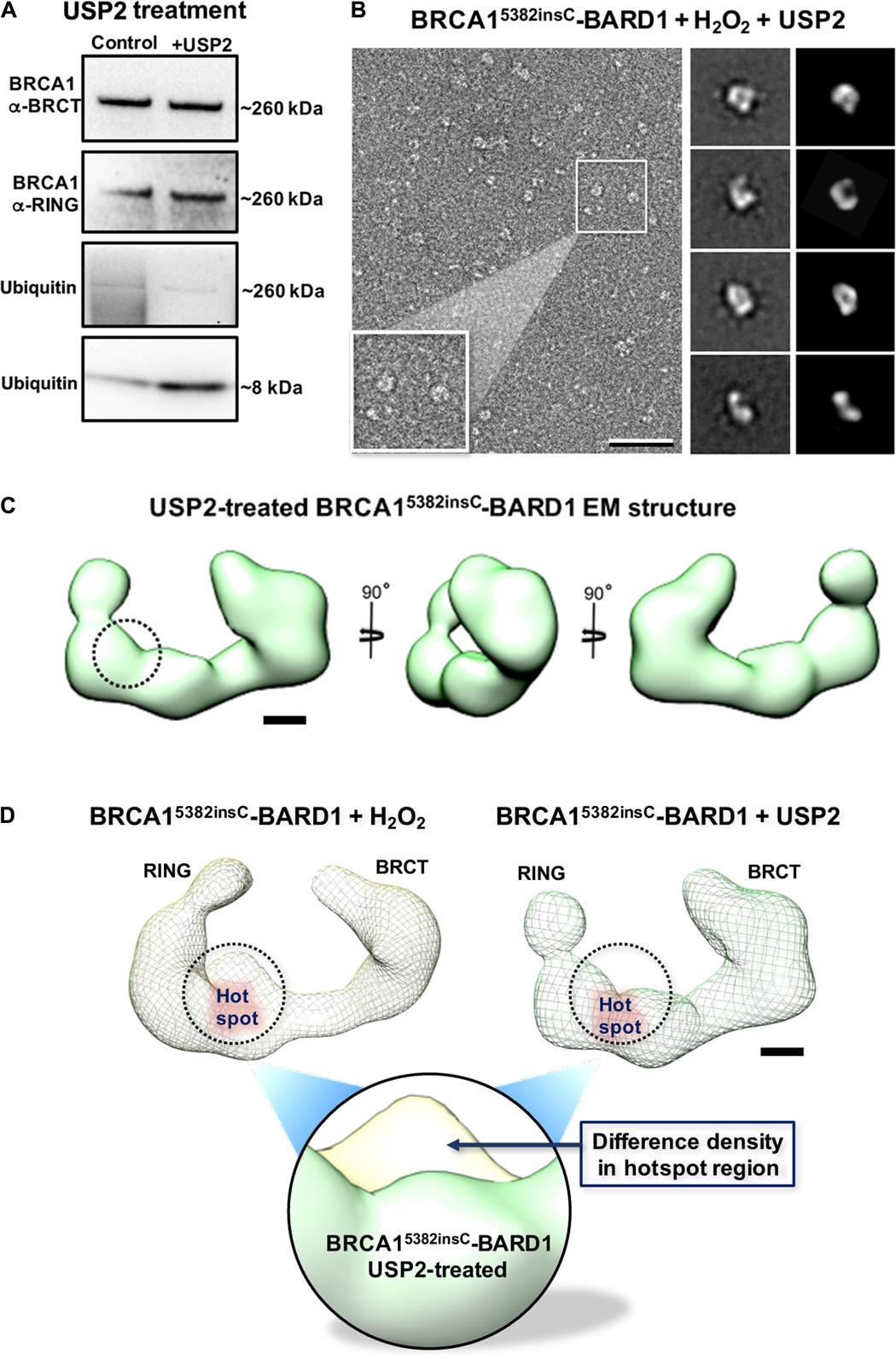
Cancer cells afflicted with mutations in the breast cancer susceptibility protein (BRCA1) often suffer from increased DNA damage and genomic instability. The precise manner in which physical changes to BRCA1 influence its role in DNA maintenance remains unclear. We used single-particle electron microscopy to study the three-dimensional properties of BRCA1 naturally produced in breast cancer cells. Structural studies revealed new information for full-length BRCA1, engaging its nuclear binding partner, the BRCA1-associated RING domain protein (BARD1). Equally important, we identified a region in mutated BRCA1 that was highly susceptible to ubiquitination. We refer to this site as a modification “hotspot.” Ubiquitin adducts in the hotspot region proved to be biochemically reversible. Collectively, we show how key changes to BRCA1 affect its structure-function relationship, and present new insights to potentially modulate mutated BRCA1 in human cancer cells.
BRCA1 的结构分析揭示了修饰热点
乳腺癌易感蛋白(BRCA1)发生突变后,癌细胞的 DNA 损伤和基因组不稳定性往往会增加。BRCA1 的物理变化对其 DNA 维护作用的确切影响方式仍不清楚。我们利用单粒子电子显微镜研究了乳腺癌细胞中天然产生的 BRCA1 的三维特性。结构研究揭示了全长 BRCA1 与其核结合伙伴 BRCA1 相关 RING 结构域蛋白(BARD1)结合的新信息。同样重要的是,我们在突变的 BRCA1 中发现了一个极易被泛素化的区域。我们将该区域称为修饰 "热点"。事实证明,热点区域的泛素加合物在生物化学上是可逆的。总之,我们展示了 BRCA1 的关键变化是如何影响其结构-功能关系的,并提出了可能调节人类癌细胞中突变 BRCA1 的新见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


