Neutrophil extracellular traps in acrolein promoted hepatic ischemia reperfusion injury: Therapeutic potential of NOX2 and p38MAPK inhibitors
Abstract
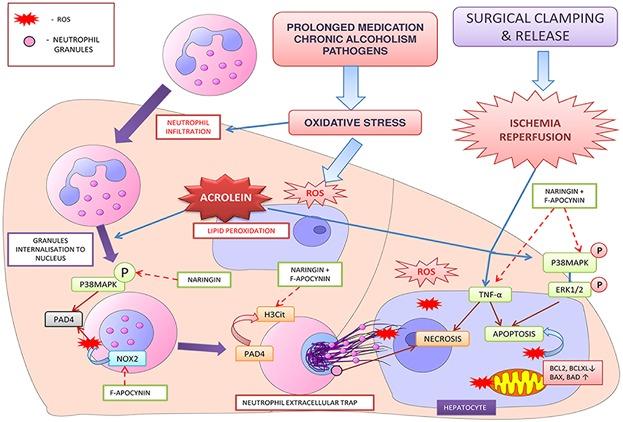
Neutrophil is a significant contributor to ischemia reperfusion (IR) induced liver tissue damage. However, the exact role of neutrophils in IR induced innate immune activation and liver damage is not quite clear. Our study sheds light on the role of chronic oxidative stress end products in worsening the IR inflammatory process by neutrophil recruitment and activation following liver surgery. We employed specific inhibitors for molecular targets—NOX2 (NADPH oxidase 2) and P38 MAPK (Mitogen activated protein kinase) signal to counteract neutrophil activation and neutrophil extracellular trap (NET) release induced liver damage in IR injury. We found that acrolein initiated neutrophil chemotaxis and induced NET release both in vitro and in vivo. Acrolein exposure caused NET induced nuclear and mitochondrial damage in HepG2 cells as well as aggravated the IR injury in rat liver. Pretreatment with F-apocynin and naringin, efficiently suppressed acrolein induced NET release in vitro. Notably, it suppressed the expression of inflammatory cytokines, P38MAPK-ERK activation, and apoptotic signals in rat liver exposed to acrolein and subjected to IR. Moreover, this combination effectively attenuated acrolein induced NET release and hepatic IR injury. In the current study we have shown that the acrolein accumulation in liver due to chronic stress, is responsible for neutrophil recruitment and its activation leading to NET induced liver damage during surgery. Our study shows that therapeutic targeting of NOX2 and P38MAPK signaling in patients with chronic hepatic disorders would improve post operative hepatic function and survival.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


