Heterochronic microRNAs in temporal specification of neural stem cells: application toward rejuvenation
IF 4.1
Q2 GERIATRICS & GERONTOLOGY
引用次数: 8
Abstract
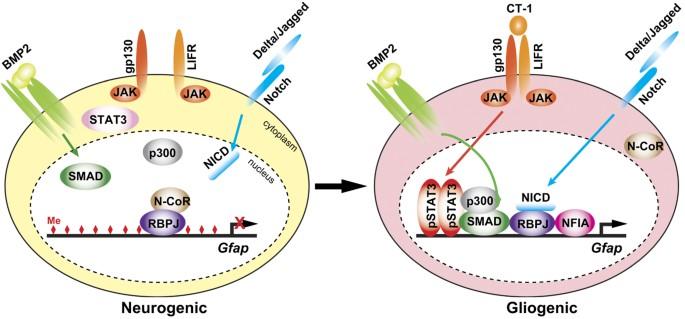
Plasticity is a critical factor enabling stem cells to contribute to the development and regeneration of tissues. In the mammalian central nervous system (CNS), neural stem cells (NSCs) that are defined by their capability for self-renewal and differentiation into neurons and glia, are present in the ventricular neuroaxis throughout life. However, the differentiation potential of NSCs changes in a spatiotemporally regulated manner and these cells progressively lose plasticity during development. One of the major alterations in this process is the switch from neurogenesis to gliogenesis. NSCs initiate neurogenesis immediately after neural tube closure and then turn to gliogenesis from midgestation, which requires an irreversible competence transition that enforces a progressive reduction of neuropotency. A growing body of evidence indicates that the neurogenesis-to-gliogenesis transition is governed by multiple layers of regulatory networks consisting of multiple factors, including epigenetic regulators, transcription factors, and non-coding RNA (ncRNA). In this review, we focus on critical roles of microRNAs (miRNAs), a class of small ncRNA that regulate gene expression at the post-transcriptional level, in the regulation of the switch from neurogenesis to gliogenesis in NSCs in the developing CNS. Unraveling the regulatory interactions of miRNAs and target genes will provide insights into the regulation of plasticity of NSCs, and the development of new strategies for the regeneration of damaged CNS.
神经干细胞时间规范中的异时性微RNA:在返老还童中的应用
可塑性是干细胞促进组织发育和再生的关键因素。在哺乳动物中枢神经系统(CNS)中,神经干细胞(NSCs)具有自我更新和分化为神经元和胶质细胞的能力,终生存在于脑室神经轴中。然而,NSCs 的分化潜能会以时空调控的方式发生变化,这些细胞在发育过程中会逐渐丧失可塑性。这一过程中的主要变化之一就是从神经发生转变为胶质细胞生成。神经干细胞在神经管闭合后立即启动神经发生,然后从妊娠中期开始转向胶质细胞发生,这需要一个不可逆的能力转换过程,从而导致神经能力的逐步降低。越来越多的证据表明,神经发生向神经胶质细胞发生的转变受多层调控网络的支配,这些网络由多种因素组成,包括表观遗传调控因子、转录因子和非编码 RNA(ncRNA)。在这篇综述中,我们将重点讨论微小RNA(miRNA)在调控发育中中枢神经系统NSCs从神经发生向神经胶质生成转换过程中的关键作用,miRNA是一类在转录后水平调控基因表达的小型ncRNA。揭示 miRNA 与靶基因之间的调控相互作用将有助于深入了解 NSCs 的可塑性调控,并为受损中枢神经系统的再生开发新的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


