Role of the central nervous system and adipose tissue BDNF/TrkB axes in metabolic regulation
IF 4.1
Q2 GERIATRICS & GERONTOLOGY
引用次数: 42
Abstract
Brain-derived neurotrophic factor (BDNF) and its receptor (tropomyosin-related kinase B: TrkB, also known as Ntrk2) have a key role in central regulation of the energy balance. BDNF and TrkB are also expressed in the peripheral tissues, including adipose tissue, but their peripheral role has been unclear. Here we report on the functional significance of the adipose tissue BDNF/TrkB axis in metabolic homeostasis. To examine the role of the BDNF/TrkB axis in the central nervous system and in adipose tissue, we generated adipocyte-specific or neuron-specific BDNF/TrkB conditional knockout (CKO) mice. Then we compared the feeding behavior and metabolic profile between each type of CKO mouse and their littermates. Bdnf expression was significantly increased in the adipose tissue of mice receiving a high-calorie diet, whereas Ntrk2 expression was decreased. The Bdnf/Ntrk2 expression ratio of adipose tissue was higher in female mice than male mice. Fabp4-Cre mice are widely used to establish adipocyte-specific CKO mice. However, we found that Fabp4-Cre-induced deletion of Bdnf or Ntrk2 led to hyperphagia, obesity, and aggressiveness, presumably due to ectopic Fabp4-Cre mediated gene recombination in the brain. Next, we attempted to more specifically delete Bdnf or Ntrk2 in adipocytes using Adipoq-Cre mice. Expression of Ntrk2, but not Bdnf, in the adipose tissue was reduced by Adipoq-Cre mediated gene recombination, indicating that adipocytes only expressed TrkB. No phenotypic changes were detected when Adipoq-Cre TrkB CKO mice were fed a normal diet, whereas female CKO mice receiving a high-calorie diet showed a decrease in food intake and resistance to obesity. The adipose tissue BDNF/TrkB axis has a substantial influence on the feeding behavior and obesity in female mice. A signaling pathway that inhibits appetite in the brain plays an opposite role in fat tissue, with a particularly strong effect in females. Within the brain, the interaction between brain-derived neurotrophic factor (BDNF) and tropomyosin-related kinase B (TrkB) promotes weight loss and reduced eating. Researchers led by Tohru Minamino at Niigata University in Japan have demonstrated that these proteins exhibit a different activity in fat. Whereas mice lacking BDNF or TrkB in the brain were prone to overeating and obesity, these traits were absent when this pathway was disrupted in fat. Intriguingly, female mice lacking TrkB in their fat cells proved less prone to obesity when fed a high-fat diet, while little change was seen in males. This may result from interactions with estrogen signaling, and the researchers see a potential target for treating female obesity.
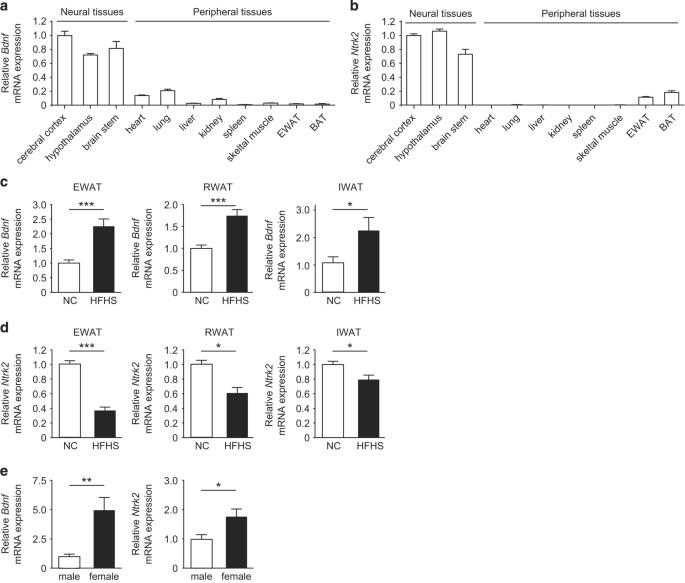
中枢神经系统和脂肪组织 BDNF/TrkB 轴在代谢调节中的作用
脑源性神经营养因子(BDNF)及其受体(肌钙蛋白相关激酶 B:TrkB,又称 Ntrk2)在能量平衡的中枢调节中起着关键作用。BDNF 和 TrkB 也在包括脂肪组织在内的外周组织中表达,但它们在外周组织中的作用尚不清楚。在此,我们报告了脂肪组织 BDNF/TrkB 轴在代谢平衡中的功能意义。为了研究 BDNF/TrkB 轴在中枢神经系统和脂肪组织中的作用,我们产生了脂肪细胞特异性或神经元特异性 BDNF/TrkB 条件性基因敲除(CKO)小鼠。然后,我们比较了每种 CKO 小鼠与同窝小鼠的摄食行为和代谢情况。在接受高热量饮食的小鼠脂肪组织中,Bdnf的表达明显增加,而Ntrk2的表达则有所下降。雌性小鼠脂肪组织中Bdnf/Ntrk2的表达比例高于雄性小鼠。Fabp4-Cre小鼠被广泛用于建立脂肪细胞特异性CKO小鼠。但我们发现,Fabp4-Cre诱导的Bdnf或Ntrk2缺失会导致多食、肥胖和攻击性,这可能是由于Fabp4-Cre介导的基因在大脑中异位重组所致。接下来,我们尝试使用 Adipoq-Cre 小鼠更特异地删除脂肪细胞中的 Bdnf 或 Ntrk2。Adipoq-Cre 介导的基因重组减少了脂肪组织中 Ntrk2 的表达,但没有减少 Bdnf 的表达,这表明脂肪细胞只表达 TrkB。给 Adipoq-Cre TrkB CKO 小鼠喂食正常饮食时,未发现表型变化,而给雌性 CKO 小鼠喂食高热量饮食时,其食物摄入量会减少,并表现出肥胖抵抗力。脂肪组织 BDNF/TrkB 轴对雌性小鼠的摄食行为和肥胖有很大影响。大脑中抑制食欲的信号通路在脂肪组织中起着相反的作用,对雌性小鼠的影响尤其大。在大脑中,脑源性神经营养因子(BDNF)和肌球蛋白相关激酶B(TrkB)之间的相互作用促进了体重减轻和进食减少。日本新泻大学的南野藤鲁领导的研究人员证明,这些蛋白质在脂肪中表现出不同的活性。大脑中缺乏 BDNF 或 TrkB 的小鼠容易暴饮暴食和肥胖,而当脂肪中的这一通路被破坏时,这些特征就不存在了。耐人寻味的是,脂肪细胞中缺乏 TrkB 的雌性小鼠在喂食高脂肪饮食时不易肥胖,而雄性小鼠则几乎没有变化。这可能是与雌激素信号相互作用的结果,研究人员发现了治疗女性肥胖症的潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


