In Vitro and Ex Vivo Approaches to Evaluate Next-Generation Tobacco and Non-Tobacco Products on Human Blood Platelets.
Q2 Health Professions
引用次数: 1
Abstract
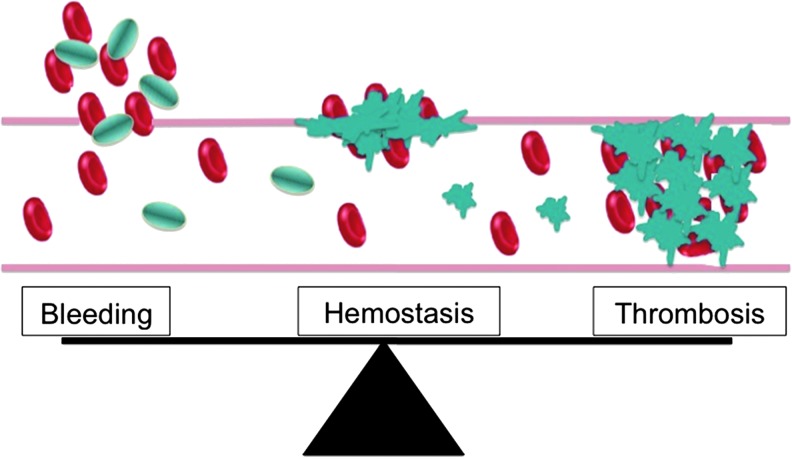
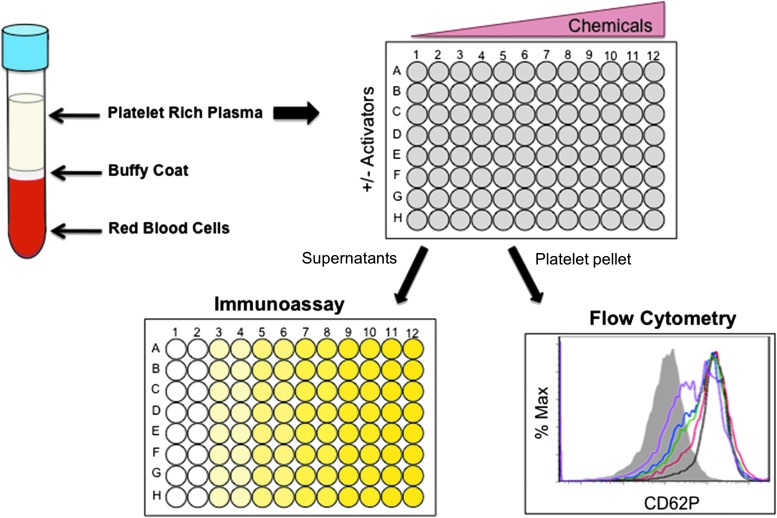
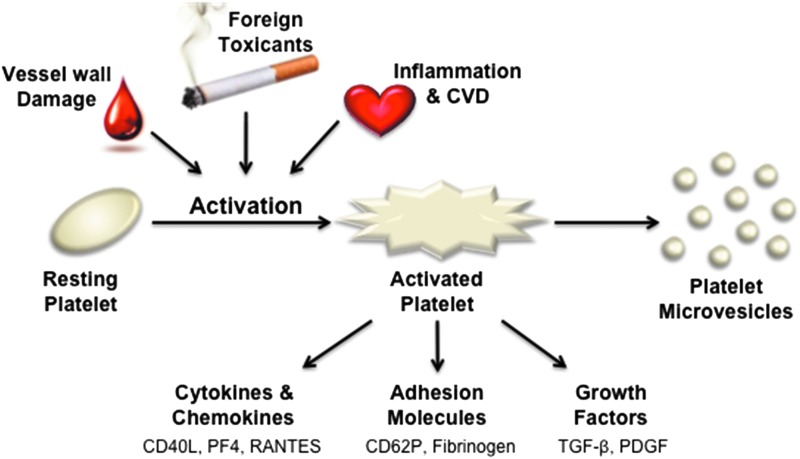
Abstract Human blood platelets are major hemostatic regulators in the circulation and important in the mediation of chronic inflammation and immunomodulation. They are key elements that promote cardiovascular pathogenesis that leads to atherosclerosis, thrombosis, myocardial infarction, and stroke. New information on tobacco use and platelet dysregulation shows that these highly understudied vascular cells are dysregulated by tobacco smoke. Thus, platelet function studies should be an important consideration for the evaluation of existing and next-generation tobacco and non-tobacco products. Novel in vitro approaches are being sought to investigate these products and their influence on platelet function. Platelets are ideally suited for product assessment, as robust and novel in vitro translational methods are available to assess platelet function. Furthermore, the use of human biological systems has the advantage that risk predictions will better reflect the human condition.
体外和离体方法评估下一代烟草和非烟草制品对人血小板的影响。
血小板是血液循环中主要的止血调节剂,在慢性炎症和免疫调节中起重要作用。它们是促进心血管发病的关键因素,导致动脉粥样硬化、血栓形成、心肌梗死和中风。关于烟草使用和血小板失调的新信息表明,这些高度未被充分研究的血管细胞受到烟草烟雾的失调。因此,血小板功能研究应该是评估现有和下一代烟草和非烟草制品的重要考虑因素。正在寻求新的体外方法来研究这些产品及其对血小板功能的影响。血小板非常适合产品评估,因为稳健和新颖的体外翻译方法可用于评估血小板功能。此外,使用人类生物系统的优势在于,风险预测将更好地反映人类的状况。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied In Vitro Toxicology
Health Professions-Medical Laboratory Technology
CiteScore
2.70
自引率
0.00%
发文量
13
期刊介绍:
Applied In Vitro Toxicology is a peer-reviewed journal providing the latest research on the application of alternative in vitro testing methods for predicting adverse effects in the pharmaceutical, chemical, and personal care industries. This Journal aims to address important issues facing the various chemical industries, including regulatory requirements; the reduction, refinement, and replacement of animal testing; new screening methods; evaluation of new cell and tissue models; and the most appropriate methods for assessing safety and satisfying regulatory demands. The Journal also delivers the latest views and opinions of developers of new models, end users of the models, academic laboratories that are inventing new tools, and regulatory agencies in the United States, Europe, Latin America, Australia and Asia. Applied In Vitro Toxicology is the journal that scientists involved with hazard identification and risk assessment will read to understand how new and existing in vitro methods are applied, and the questions for which these models provide answers. Applied In Vitro Toxicology coverage includes: -Applied in vitro toxicology industry standards -New technologies developed for applied in vitro toxicology -Data acquisition, cleaning, distribution, and best practices -Data protection, privacy, and policy -Business interests from research to product -The changing role of in vitro toxicology -Visualization and design principles of applied in vitro toxicology infrastructures -Physical interfaces and robotics -Opportunities around applied in vitro toxicology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


