Photophysical and electrochemical properties of 9-naphthyl-3,6-diaminocarbazole derivatives and their application as photosensitizers
IF 3.261
引用次数: 1
Abstract
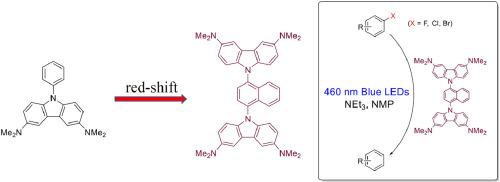
A series of 3,6-diamino-9-naphthylcarbazole derivatives were synthesized and characterized experimentally and computationally. As the lowest unoccupied molecular orbital of the naphthyl group has lower energy than that of the phenyl group, a charge transfer from carbazole to naphthyl in the excited states occurred causing solvatofluorochromism and solvent-dependency in fluorescence quantum yields. A molecule having two carbazole substituents sandwiching the central naphthyl ring had absorption reaching 470 nm and a high reducing capability in the excited state. This molecule could successfully photosensitize the hydrodehalogenation of haloarenes under visible light irradiation.
9-萘-3,6-二氨基咔唑衍生物的光物理和电化学性质及其作为光敏剂的应用
合成了一系列3,6-二氨基-9-萘基咔唑衍生物,并进行了实验和计算表征。由于萘基的最低未占据分子轨道比苯基的能量低,在激发态下,咔唑向萘基发生电荷转移,导致荧光量子产率的溶剂荧光变色和溶剂依赖性。在激发态下,两个咔唑取代基夹在中心环烷环上的分子具有高达470 nm的吸收和高还原能力。该分子能在可见光照射下成功地光敏卤代烃的加氢脱卤反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


