Copper oxide and manganese dioxide nanoparticles on corrugated glass-fiber supporters promote thermocatalytic oxidation of formaldehyde
Abstract
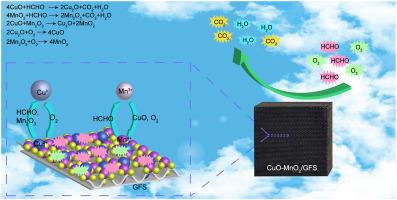
Low catalytic activity and difficult separation of manganese dioxide nanoparticles limit their application in the removal of volatile organic compounds (VOCs). Herein, copper oxide (CuO) and δ-manganese dioxide (MnO2) catalysts were deposited uniformly on a glass-fiber supporter (GFS) by an in-situ precipitation method. The GFS with the hierarchical pores and great specific surface areas was employed as a catalytic supporter. The MnO2 major catalysts and CuO cocatalysts synergistically promoted the formaldehyde thermocatalytic oxidation, and the removal efficiency arrived at nearly 100% even after cycle tests of 4 times. Thermodynamic calculation results demonstrated that the formaldehyde thermocatalytic oxidation over the CuO–MnO2/GFS was performed via a Mars-van Krevelen mechanism. Namely, the formaldehyde molecules were oxidized by the copper oxide and δ-manganese dioxide, and then the as-produced cuprous oxide and manganese sesquioxide were re-oxidized into copper oxide and manganese dioxide by gas phase oxygen. Notably, copper oxide cocatalysts significantly improved the catalytic activity of the CuO–MnO2/GFS because they not only directly participated in the formaldehyde oxidation but also promoted the oxidation reaction of manganese sesquioxide into manganese oxide. This work revealed the synergistic mechanism of MnO2 major catalysts and CuO cocatalysts in promoting thermocatalytic oxidation of formaldehyde, and provided a promising thermocatalyst for the purification of industrial waste gas.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


