High-intensity ultrasound combined with glycation enhances the thermal stability and in vitro digestion behaviors of myofibrillar protein aqueous solution
Abstract
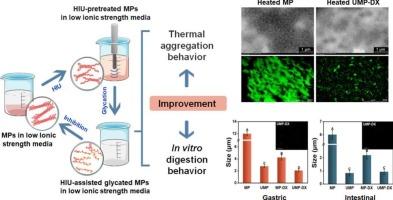
The low thermal stability of myofibrillar proteins (MPs) is a technological barrier to them being applied in beverage formulas. In this study, we investigated the effect of high-intensity ultrasound (HIU) pretreatment combined with glycation on the thermal stability, structural characteristics, and in vitro digestion behavior of MPs in water. The results indicated that HIU pretreatment combined with glycation significantly inhibited thermal aggregation and reduced the particle size of MPs compared to using either HIU or glycation treatments individually. The grafting of dextran (DX) shielded the sulfhydryl (–SH) and hydrophobic groups and inhibited disulfide bond cross-linking and hydrophobic association. Moreover, HIU pretreatment facilitated the shielding effect of glycation by destroying the filamentous myosin structure and exposing the internal –SH and hydrophobic groups as well as the grafting sites, maximally inhibiting thermal aggregation. In addition, the smaller protein particles and more flexible structure caused by HIU pretreatment combined with glycation increased their binding affinity toward protease. Overall, these findings can promote the technological development of modulating the MP structure–digestion for formulating novel meat protein-based products.
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|---|---|
| Sigma |
trypsin
|
¥28.00~¥311508.69 |
| Sigma |
pepsin
|
¥32.00~¥98370.53 |
| Sigma |
trichloroacetic acid
|
¥21.00~¥8553.00 |
| 阿拉丁 |
porcine longissimus muscle
|
¥2021.04~¥2021.04 |
| 上海源叶 |
DX
|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


