Clinical Performance of Rapid Antigen Tests for the Detection of SARS-CoV-2 Infection in the Emergency Department and Community: A Retrospective Study.
The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale
Pub Date : 2022-10-06
eCollection Date: 2022-01-01
DOI:10.1155/2022/9447251
引用次数: 1
Abstract
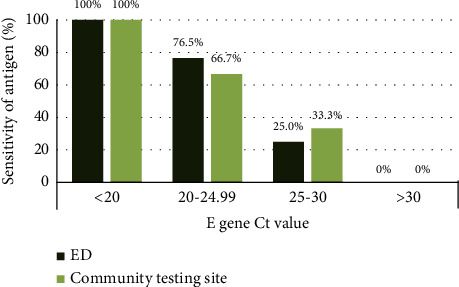
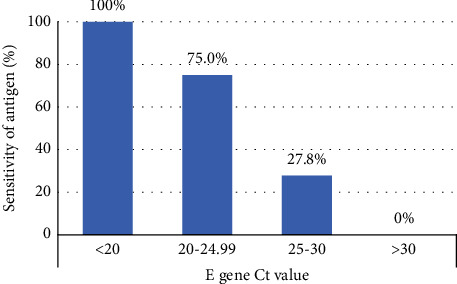
Background Rapid antigen tests for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection have been authorized for emergency use (EUA); however, the performance has not been fully evaluated in clinical contexts. This study aimed to provide evidence regarding the diagnostic performance of SARS-CoV-2 rapid antigen tests compared with the real-time reverse transcription-polymerase chain reaction (RT-PCR) test in the emergency department (ED) and community. Methods Patients who underwent SARS-CoV-2 rapid antigen tests using the VTRUST COVID-19 Antigen Rapid Test (TD-4531) and real-time RT-PCR on the same day in the ED or community from May 24, 2021, to June 24, 2021, were examined. Results Paired nasopharyngeal swabs were collected from 4022 suspected COVID-19 patients: 800 in the ED and 3222 in the community. Overall, 62 (1.54%) tested positive, 13 tested indeterminate, and 3947 tested negative by real-time RT-PCR. The sensitivity and specificity of the antigen test were 51.61% and 99.44% (overall), 62.50% and 99.61% (ED), and 31.82% and 99.40% (community), respectively. There were 30 false negatives and 22 false positives. Among the false negatives, 16.67% had a cycle threshold (Ct) value of <25. Conclusion The VTRUST COVID-19 Antigen Rapid Test showed comparable specificity as real-time RT-PCR for the ED and community, but the sensitivity was relatively low, especially when the Ct value was >25. This test can be useful for the rapid identification of infected subjects in an epidemic situation.
快速抗原试验在急诊科和社区检测SARS-CoV-2感染的临床效果:回顾性研究
背景:用于检测严重急性呼吸综合征冠状病毒2 (SARS-CoV-2)的快速抗原试验已被批准用于紧急使用(EUA);然而,在临床环境下的表现尚未得到充分评估。本研究旨在为SARS-CoV-2快速抗原检测与实时逆转录聚合酶链反应(RT-PCR)检测在急诊科(ED)和社区的诊断效能提供证据。方法:对2021年5月24日至2021年6月24日在急诊科或社区当天使用VTRUST COVID-19抗原快速检测试剂盒(TD-4531)和实时RT-PCR进行SARS-CoV-2快速抗原检测的患者进行检测。结果:收集到疑似COVID-19患者配对鼻咽拭子4022例,其中急诊科800例,社区3222例。总的来说,62例(1.54%)检测为阳性,13例检测不确定,3947例检测为阴性。抗原检测的敏感性为51.61%、99.44%(总体),特异性为62.50%、99.61% (ED), 31.82%、99.40%(社区)。有30个假阴性和22个假阳性。结论:VTRUST COVID-19抗原快速检测对ED和社区的特异性与实时RT-PCR相当,但敏感性相对较低,特别是当Ct值>25时。在传染病流行的情况下,这一测试可用于快速识别受感染的受试者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


