Alternative Synthesis and Structures of C-monoacetylenic Phosphaalkenes.
IF 1.1
4区 化学
Q4 CHEMISTRY, INORGANIC & NUCLEAR
Zeitschrift fur Anorganische und Allgemeine Chemie
Pub Date : 2012-11-01
Epub Date: 2012-10-09
DOI:10.1002/zaac.201200324
引用次数: 15
Abstract
An alternative synthesis of C-monoacetylenic phosphaalkenes trans-Mes*P=C(Me)(C≡CR) (Mes* = 2, 4, 6-tBu3Ph, R = Ph, SiMe3) from C-bromophosphaalkenes cis-Mes*P=C(Me)Br using standard Sonogashira coupling conditions is described. Crystallographic studies confirm cis-trans isomerization of the P=C double bond during Pd-catalyzed cross coupling, leading exclusively to trans-acetylenic phosphaalkenes. Crystallographic studies of all synthesized compounds reveal the extend of π-conjugation over the acetylene and P=C π-systems.
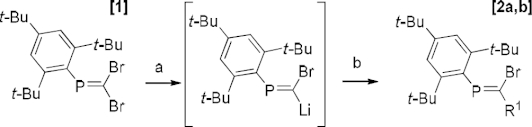
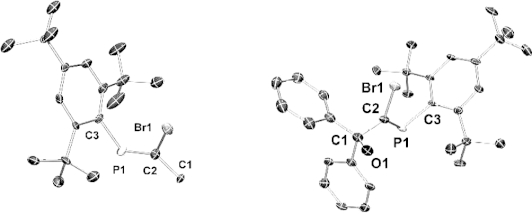
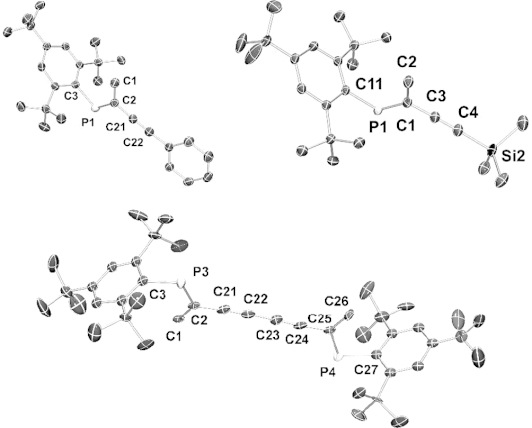
c -单乙基磷酸烯烃的替代合成及其结构。
描述了用标准Sonogashira偶联条件由C-溴代磷烯烃顺式-Mes*P=C(Me)(C≡CR)合成C-单乙基磷烯烃反式Mes*P=C(Me)(C≡CR) (Mes* = 2,4,6 - tbu3ph, R = Ph, SiMe3)。晶体学研究证实,在pd催化的交叉偶联过程中,P=C双键发生了顺-反异构化反应,只产生了反式乙炔磷酸烯烃。所有合成化合物的晶体学研究表明π共轭作用在乙炔和P=C π共轭体系上的扩展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
2.60
自引率
14.30%
发文量
308
审稿时长
1 months
期刊介绍:
ZAAC is an international scientific journal which publishes original papers on new relevant research results from all areas of inorganic chemistry, solid state chemistry, and co-ordination chemistry.
The contributions reflect the latest findings in these research areas and serve the development of new materials, such as super-hard materials, electrical superconductors, or intermetallic compounds. Up-to-date physical methods for the characterization of new chemical compounds and materials are also described.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


