Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 527
Abstract
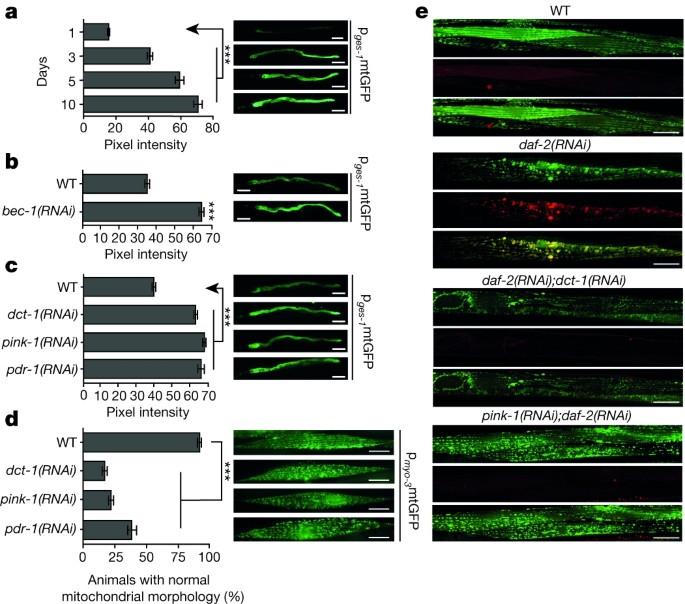
Mitophagy, a selective type of autophagy targeting mitochondria for degradation, interfaces with mitochondrial biogenesis to regulate mitochondrial content and longevity in Caenorhabditis elegans. An increase of cellular mitochondrial content is characteristic of ageing and numerous pathological conditions in humans, but the underlying cellular and molecular mechanisms have remained unclear. This study shows that the biogenesis and turnover of mitochondria are coupled in Caenorhabditis elegans. Impairment of mitophagy, which removes damaged mitochondria, reduces the worm''s stress resistance and triggers mitochondrial retrograde signalling through the SKN-1 transcription factor. SKN-1 not only regulates the expression of mitochondrial biogenesis genes but also enhances expression of DCT-1, a key regulator of mitophagy. Uncoupling of these two processes during ageing contributes to the accumulation of damaged mitochondria and decline of cellular function. Impaired mitochondrial maintenance in disparate cell types is a shared hallmark of many human pathologies and ageing1,2,3,4,5,6,7,8. How mitochondrial biogenesis coordinates with the removal of damaged or superfluous mitochondria to maintain cellular homeostasis is not well understood. Here we show that mitophagy, a selective type of autophagy targeting mitochondria for degradation, interfaces with mitochondrial biogenesis to regulate mitochondrial content and longevity in Caenorhabditis elegans. We find that DCT-1 is a key mediator of mitophagy and longevity assurance under conditions of stress in C. elegans. Impairment of mitophagy compromises stress resistance and triggers mitochondrial retrograde signalling through the SKN-1 transcription factor that regulates both mitochondrial biogenesis genes and mitophagy by enhancing DCT-1 expression. Our findings reveal a homeostatic feedback loop that integrates metabolic signals to coordinate the biogenesis and turnover of mitochondria. Uncoupling of these two processes during ageing contributes to overproliferation of damaged mitochondria and decline of cellular function.
优雅小鼠衰老过程中有丝分裂和线粒体生物生成的协调作用
线粒体吞噬是一种以线粒体降解为目标的选择性自噬类型,它与线粒体生物发生相互作用,调节线粒体含量和秀丽隐杆线虫的寿命。细胞线粒体含量的增加是人类衰老和多种病理状况的特征,但其潜在的细胞和分子机制仍不清楚。这项研究表明,线粒体的生物生成和更替在秀丽隐杆线虫中是耦合的。线粒体吞噬能清除受损的线粒体,而线粒体吞噬功能受损会降低虫体的抗应激能力,并通过 SKN-1 转录因子触发线粒体逆行信号。SKN-1 不仅能调节线粒体生物生成基因的表达,还能增强 DCT-1 的表达,DCT-1 是有丝分裂的关键调节因子。衰老过程中这两个过程的脱钩会导致受损线粒体的积累和细胞功能的衰退。不同类型细胞的线粒体维护受损是许多人类病症和衰老的共同特征1,2,3,4,5,6,7,8。线粒体生物生成如何与清除受损或多余线粒体以维持细胞平衡相协调,目前尚不十分清楚。在这里,我们发现线粒体吞噬(一种选择性自噬,以线粒体降解为目标)与线粒体生物发生相互作用,调节线粒体含量和秀丽隐杆线虫的寿命。我们发现,在胁迫条件下,DCT-1 是线粒体吞噬和保证 elegans 寿命的关键介质。线粒体吞噬功能受损会损害抗应激能力,并通过 SKN-1 转录因子触发线粒体逆行信号,而 SKN-1 转录因子通过增强 DCT-1 的表达来调控线粒体生物发生基因和线粒体吞噬功能。我们的研究结果揭示了一个平衡反馈回路,它整合了代谢信号,以协调线粒体的生物生成和周转。衰老过程中这两个过程的脱钩会导致受损线粒体的过度增殖和细胞功能的下降。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


