The direct arylation of allylic sp3 C–H bonds via organic and photoredox catalysis
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 323
Abstract
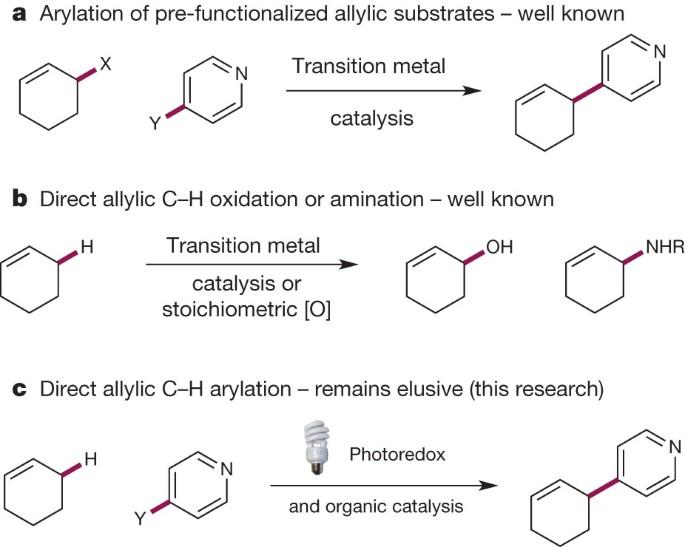
Photoredox and organic catalysis are combined to achieve broadly effective direct arylation of allylic carbon–hydrogen bonds under mild conditions; this carbon–carbon bond forming reaction readily accommodates a wide range of alkene and electron-deficient arene coupling partners. James Cuthbertson and David MacMillan describe the development of a new and general strategy for the direct arylation of allylic sp3 C–H bonds, potentially providing a strategy for the construction of complex organic molecules via the coupling of simple and otherwise inert building blocks, without introducing extraneous functional groups. The process, combing photoredox and thiol-based organic catalysis, requires only under mild conditions; and can accommodate a wide range of alkene and electron-deficient arene coupling partners. The direct functionalization of unactivated sp3 C–H bonds is still one of the most challenging problems facing synthetic organic chemists. The appeal of such transformations derives from their capacity to facilitate the construction of complex organic molecules via the coupling of simple and otherwise inert building blocks, without introducing extraneous functional groups. Despite notable recent efforts1, the establishment of general and mild strategies for the engagement of sp3 C–H bonds in C–C bond forming reactions has proved difficult. Within this context, the discovery of chemical transformations that are able to directly functionalize allylic methyl, methylene and methine carbons in a catalytic manner is a priority. Although protocols for direct oxidation and amination of allylic C–H bonds (that is, C–H bonds where an adjacent carbon is involved in a C = C bond) have become widely established2,3, the engagement of allylic substrates in C–C bond forming reactions has thus far required the use of pre-functionalized coupling partners4. In particular, the direct arylation of non-functionalized allylic systems would enable access to a series of known pharmacophores (molecular features responsible for a drug’s action), though a general solution to this long-standing challenge remains elusive. Here we report the use of both photoredox and organic catalysis to accomplish a mild, broadly effective direct allylic C–H arylation. This C–C bond forming reaction readily accommodates a broad range of alkene and electron-deficient arene reactants, and has been used in the direct arylation of benzylic C–H bonds.
通过有机催化和光氧化催化实现烯丙基 sp3 C-H 键的直接芳基化
光氧化和有机催化相结合,在温和的条件下实现了烯丙基碳-氢键的广泛有效的直接芳基化反应;这种碳-碳键形成反应可轻松容纳多种烯和缺电子炔偶联伙伴。詹姆斯-卡斯伯森(James Cuthbertson)和戴维-麦克米伦(David MacMillan)描述了烯丙基 sp3 C-H 键直接芳基化的一种新的通用策略的开发情况,该策略有可能通过简单和惰性构筑基块的偶联来构建复杂的有机分子,而无需引入外来官能团。该过程结合了光氧化和基于硫醇的有机催化,只需要在温和的条件下进行;并且可以容纳多种烯类和缺电子炔类偶联伙伴。未活化 sp3 C-H 键的直接官能化仍然是合成有机化学家面临的最具挑战性的问题之一。这种转化的吸引力来自于它们能够在不引入外来官能团的情况下,通过简单和惰性结构单元的偶联来促进复杂有机分子的构建。尽管最近做出了显著的努力1 ,但在 C-C 键形成反应中掺入 sp3 C-H 键的一般温和策略的建立仍然困难重重。在这种情况下,当务之急是发现能够以催化方式直接使烯丙基甲基、亚甲基和甲基碳官能化的化学转化方法。虽然烯丙基 C-H 键(即相邻碳参与 C = C 键的 C-H 键)的直接氧化和胺化协议已广泛确立2,3,但迄今为止,烯丙基底物参与 C-C 键形成反应需要使用预官能化偶联剂4。特别是,非官能化烯丙基系统的直接芳基化将使人们能够获得一系列已知的药代因(负责药物作用的分子特征),但这一长期挑战的总体解决方案仍然遥遥无期。在此,我们报告了利用光氧化和有机催化完成温和、广泛有效的直接烯丙基 C-H 芳基化反应的情况。这种 C-C 键形成反应可轻松应对各种烯类和缺电子炔类反应物,并已用于苄基 C-H 键的直接芳基化反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


