Preparation of rare earth metal ions promoted MnOx@halloysite catalyst for highly efficient catalytic oxidation of toluene
Abstract
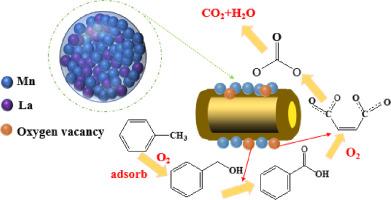
Halloysite with nanotube structure is a potential functional support to prepare high-performance catalysts for the oxidation of volatile organic compounds (VOCs) at low temperatures. In this work, rare earth metal ions promoted MnOx@halloysite system were synthesized and demonstrated improved toluene oxidation. The obtained catalyst exhibits excellent catalytic performance, including toluene conversion efficiency (T90 = 232 °C), CO2 selectivity (100%), super long-term stability and water resistance under the condition of toluene concentration with 1000 ppm. It has been demonstrated that the La-promoted halloysite-supported MnOx catalyst increased the ratio of Mn3+ and the number of surface oxygen vacancies, facilitating the formation of active oxygen species and enhancing low-temperature catalytic activity. Moreover, in situ diffuse reflectance infrared Fourier transform spectroscopy confirmed the intermediates generated during toluene oxidation. Toluene oxidation occurred via the benzyl alcohol → benzoate → anhydride reaction pathway over the obtained catalysts. This work provides a considerable experimental basis for understanding the catalytic performance and reaction mechanism of rare earth metal ions promoting the manganese oxides supported by clay minerals for toluene oxidation and paves the way for the development of high-performance catalysts toward toluene oxidation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


