The design of efficient and selective routes to pyridyl analogues of 2,3-dihydro-1,4-benzodioxin-6-carbaldehyde
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 12
Abstract
This Letter describes the synthetic routes to challenging pyridyl analogues of 2,3-dihydro-1,4-benzodioxin-6-carbaldehyde which were key intermediates for our antibacterial medicinal chemistry programme. All routes described started from kojic acid (8) and have been used to give multigram quantities of each aldehyde.
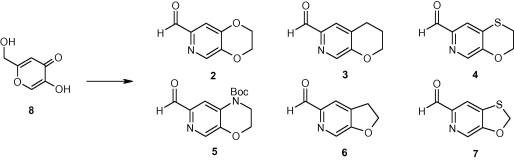
2,3-二氢-1,4-苯并二氧辛-6-乙醛吡啶基类似物的高效选择性路线设计
本信函描述了具有挑战性的2,3-二氢-1,4-苯并二氧辛-6-乙醛吡啶类似物的合成路线,这些类似物是我们抗菌药物化学计划的关键中间体。所描述的所有途径都是从曲酸(8)开始的,并已用于给出每种醛的多图量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


