An innovative hybrid hydrometallurgical approach for precious metals recovery from secondary resources
Abstract
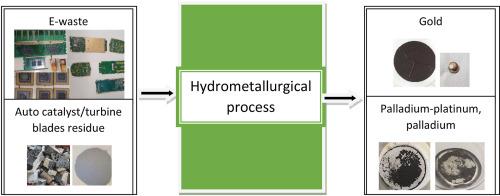
The current paper presents an innovative hydrometallurgical methodology for recovery of precious metals from different waste streams. The used hydrometallurgical process, which has been already patented (Birloaga and Francesco Veglio, 2019), consists of a single leaching system (HCl, H2O2 and C2H4O2) of all elements and then selective recovery of elements from solution by chemical reduction/processes. About 99% of Au dissolution efficiency was achieved using: 3.5 M HCl; 1.96 M H2O2; 1.67 M of C2H4O2; 5 h without stirring; room temperature; 20% of solid concentration. The same conditions have resulted recovery of over 95% of Au from spent mobile phones PCBs. Over 80% of Au was achieved by three steps of leaching of ceramic Intel CPU. The influences of hydrochloric acid concentration and process time have been evaluated for Pd and Pt leaching from spent autocatalyst. Over 89% of Pt and 100% of Pd recovery were obtained from spent catalyst using: 5 M HCl; 1.96 M H2O2; 1.67 M of C2H4O2; 250 rpm of stirring for 3 h; room temperature; 10% of solid concentration. More than 98% of Pd recovery was achieved from the turbine residue after 20 min of reaction. The almost complete recovery of Au (99%) from solution was achieved by reduction with ascorbic acid. Complete recovery of Pd and about 79% of Pt have been obtained by cementation with ion metal powder from the leaching solution of spent autocatalyst. The application of reduction process with iron metal on the solution of turbine residues led to over 99% of Pd recovery. This efficient process provides a new way to recycle precious metals and to effectively prevent environmental pollution from different e-waste and other waste streams.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


