Vertebrate protein glycosylation: diversity, synthesis and function
IF 90.2
1区 生物学
Q1 CELL BIOLOGY
引用次数: 1265
Abstract
Approximately half of human proteins are glycosylated, and the resulting diverse glycan patterns encode an additional level of information. The process of protein glycosylation is mediated by numerous enzymes with dynamic localization, regulation and specificity. High-throughput techniques facilitate the study of complex protein glycans and may give further insights into their roles in protein homeostasis, cell signalling and cell adhesion. Protein glycosylation is a ubiquitous post-translational modification found in all domains of life. Despite their significant complexity in animal systems, glycan structures have crucial biological and physiological roles, from contributions in protein folding and quality control to involvement in a large number of biological recognition events. As a result, they impart an additional level of ''information content'' to underlying polypeptide structures. Improvements in analytical methodologies for dissecting glycan structural diversity, along with recent developments in biochemical and genetic approaches for studying glycan biosynthesis and catabolism, have provided a greater understanding of the biological contributions of these complex structures in vertebrates.
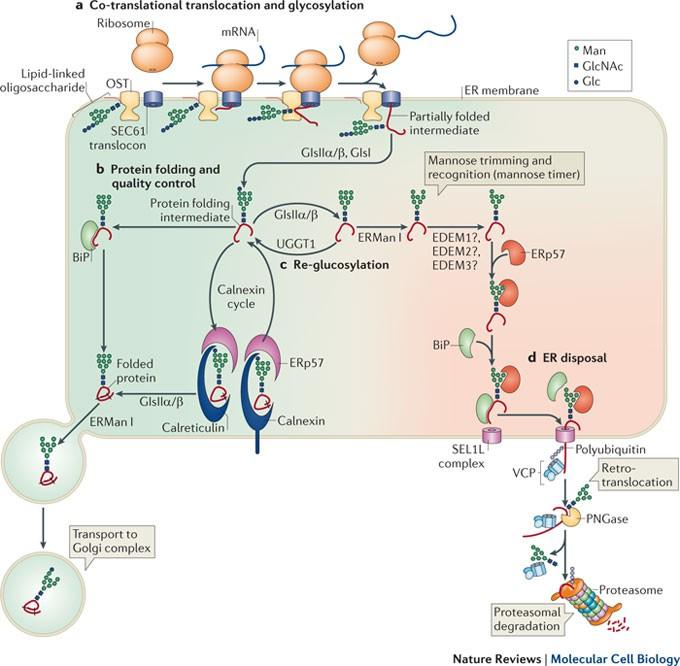
脊椎动物蛋白质糖基化:多样性、合成和功能
约有一半的人类蛋白质被糖基化,由此产生的各种聚糖模式编码了更多的信息。蛋白质糖基化过程由许多酶介导,这些酶具有动态定位、调节和特异性。高通量技术促进了对复杂蛋白质聚糖的研究,并可能进一步揭示它们在蛋白质稳态、细胞信号和细胞粘附中的作用。蛋白质糖基化是一种普遍存在于所有生命领域的翻译后修饰。尽管糖基化结构在动物系统中非常复杂,但它们在生物学和生理学方面却起着至关重要的作用,包括对蛋白质折叠和质量控制的贡献,以及参与大量的生物识别事件。因此,它们为基本的多肽结构提供了额外的 "信息含量"。剖析聚糖结构多样性的分析方法的改进,以及研究聚糖生物合成和分解的生化和遗传方法的最新发展,使人们对脊椎动物中这些复杂结构的生物学贡献有了更深入的了解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
173.60
自引率
0.50%
发文量
118
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Molecular Cell Biology is a prestigious journal that aims to be the primary source of reviews and commentaries for the scientific communities it serves. The journal strives to publish articles that are authoritative, accessible, and enriched with easily understandable figures, tables, and other display items. The goal is to provide an unparalleled service to authors, referees, and readers, and the journal works diligently to maximize the usefulness and impact of each article. Nature Reviews Molecular Cell Biology publishes a variety of article types, including Reviews, Perspectives, Comments, and Research Highlights, all of which are relevant to molecular and cell biologists. The journal's broad scope ensures that the articles it publishes reach the widest possible audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


