Synthesis of N-heterocyclic carbene ligands and derived ruthenium olefin metathesis catalysts
IF 16
1区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 144
Abstract
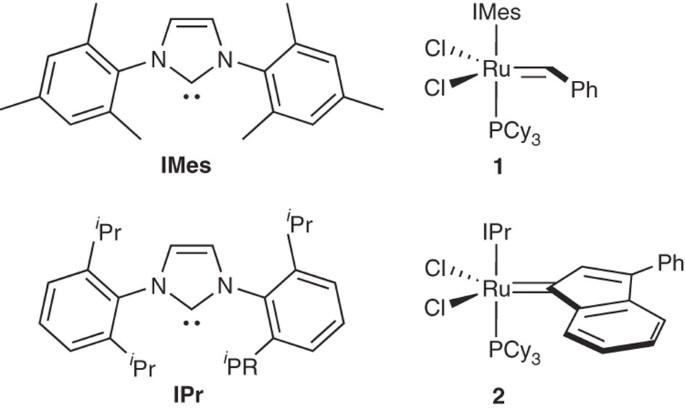
We describe the synthesis of commonly used free N-heterocyclic carbenes (NHCs), 1,3-bis-(2,4,6-trimethylphenyl)imidazol-2-ylidene (IMes) and 1,3-bis-(2,6-diisopropylphenyl)imidazol-2-ylidene (IPr), and of the two corresponding ruthenium-based metathesis complexes. The complex containing IMes was found to be highly efficient in macrocyclizations involving ring-closing metatheses (RCM), whereas the complex featuring the IPr ligand shows excellent activity in both RCM and cross metathesis because of its greater stability. The free carbenes IMes and IPr are isolated in four steps, with an overall yield of ∼50%. They are then used to replace a labile phosphine in precatalysts belonging to two families of ruthenium-containing complexes, benzylidene and indenylidene types, respectively. Such complexes are isolated as analytically pure compounds with 77% and 95% yield. The total time for the synthesis of the free NHCs is 56 h, and incorporation in complexes requires an additional 4–5 h.
合成 N-杂环碳烯配体和衍生钌烯烃偏析催化剂
我们介绍了常用游离 N-杂环碳烯(NHC)、1,3-双-(2,4,6-三甲基苯基)咪唑-2-亚基(IMes)和 1,3-双-(2,6-二异丙基苯基)咪唑-2-亚基(IPr)以及两种相应的钌基偏析配合物的合成。研究发现,含有 IMes 的配合物在涉及闭环偏析(RCM)的大环化反应中具有很高的效率,而含有 IPr 配体的配合物由于具有更高的稳定性,在 RCM 和交叉偏析反应中都表现出卓越的活性。游离碳烯 IMes 和 IPr 分四步分离,总产率为 50%。然后,它们被用来替代前催化剂中的易变膦,这两种前催化剂分别属于亚苄基和亚茚基类型的含钌络合物家族。这些配合物分离出来的分析纯度分别为 77% 和 95%。合成游离 NHC 的总时间为 56 小时,而将其加入络合物中还需要 4-5 小时。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Protocols
生物-生化研究方法
CiteScore
29.10
自引率
0.70%
发文量
128
审稿时长
4 months
期刊介绍:
Nature Protocols focuses on publishing protocols used to address significant biological and biomedical science research questions, including methods grounded in physics and chemistry with practical applications to biological problems. The journal caters to a primary audience of research scientists and, as such, exclusively publishes protocols with research applications. Protocols primarily aimed at influencing patient management and treatment decisions are not featured.
The specific techniques covered encompass a wide range, including but not limited to: Biochemistry, Cell biology, Cell culture, Chemical modification, Computational biology, Developmental biology, Epigenomics, Genetic analysis, Genetic modification, Genomics, Imaging, Immunology, Isolation, purification, and separation, Lipidomics, Metabolomics, Microbiology, Model organisms, Nanotechnology, Neuroscience, Nucleic-acid-based molecular biology, Pharmacology, Plant biology, Protein analysis, Proteomics, Spectroscopy, Structural biology, Synthetic chemistry, Tissue culture, Toxicology, and Virology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


