Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 1729
Abstract
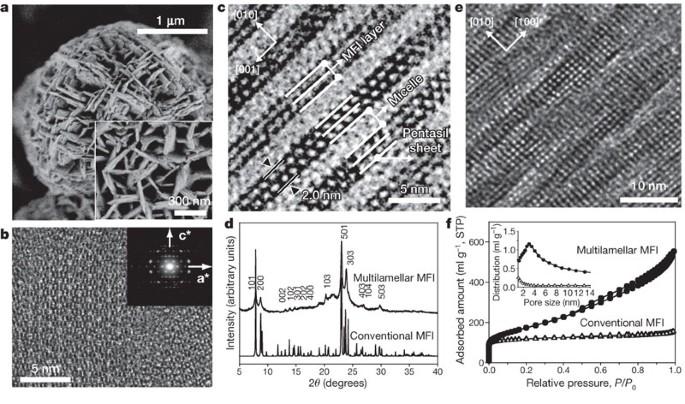
Zeolites — microporous crystalline aluminosilicates — are widely used in industry as size- and shape-selective catalysts. But the very micropores that make this catalytic activity possible also cause diffusion limitations. Choi et al. now show that the problem can be overcome by synthesizing zeolites in the presence of bifunctional surfactants, which simultaneously direct the formation of micropores and limit the growth of the zeolite crystal to that of a ''nanosheet'' with a thickness of only one unit cell. These structural features render the ultrathin zeolites highly active for the catalytic conversion of large organic molecules; they also minimize the adverse effects of diffusion limitations, as illustrated by drastically reduced coke deposition and catalyst deactivation during methanol-to-gasoline conversion. Zeolites — microporous crystalline aluminosilicates — are widely used in industry as size- and shape-selective catalysts, but the micropores that enable this catalytic activity also cause diffusion limitations that adversely affect it. This can be overcome by reducing the thickness of the zeolite crystals and thus improving molecular diffusion. Here it is shown that bifunctional surfactants can direct the formation of zeolite structures that are only one unit cell thick. Zeolites—microporous crystalline aluminosilicates—are widely used in petrochemistry and fine-chemical synthesis1,2,3 because strong acid sites within their uniform micropores enable size- and shape-selective catalysis. But the very presence of the micropores, with aperture diameters below 1 nm, often goes hand-in-hand with diffusion limitations3,4,5 that adversely affect catalytic activity. The problem can be overcome by reducing the thickness of the zeolite crystals, which reduces diffusion path lengths and thus improves molecular diffusion4,5. This has been realized by synthesizing zeolite nanocrystals6, by exfoliating layered zeolites7,8,9, and by introducing mesopores in the microporous material through templating strategies10,11,12,13,14,15,16,17 or demetallation processes18,19,20,21,22. But except for the exfoliation, none of these strategies has produced ‘ultrathin’ zeolites with thicknesses below 5 nm. Here we show that appropriately designed bifunctional surfactants can direct the formation of zeolite structures on the mesoporous and microporous length scales simultaneously and thus yield MFI (ZSM-5, one of the most important catalysts in the petrochemical industry) zeolite nanosheets that are only 2 nm thick, which corresponds to the b-axis dimension of a single MFI unit cell. The large number of acid sites on the external surface of these zeolites renders them highly active for the catalytic conversion of large organic molecules, and the reduced crystal thickness facilitates diffusion and thereby dramatically suppresses catalyst deactivation through coke deposition during methanol-to-gasoline conversion. We expect that our synthesis approach could be applied to other zeolites to improve their performance in a range of important catalytic applications.
稳定的沸石 MFI 单胞纳米片作为活性长寿命催化剂
沸石--微孔结晶铝硅酸盐--作为尺寸和形状选择性催化剂被广泛应用于工业领域。但是,使这种催化活性成为可能的微孔也造成了扩散限制。Choi 等人的研究表明,可以通过在双功能表面活性剂存在下合成沸石来克服这一问题。双功能表面活性剂可以同时引导微孔的形成,并将沸石晶体的生长限制为厚度仅为一个单元格的 "纳米片"。这些结构特征使超薄沸石在催化转化大分子有机物时具有很高的活性;它们还能将扩散限制的不利影响降到最低,在甲醇转化为汽油的过程中,焦炭沉积和催化剂失活现象大幅减少就说明了这一点。沸石--微孔结晶铝硅酸盐--在工业中被广泛用作尺寸和形状选择性催化剂,但使这种催化活性得以实现的微孔也会造成扩散限制,从而对催化活性产生不利影响。可以通过减小沸石晶体的厚度从而改善分子扩散来克服这一问题。这里的研究表明,双功能表面活性剂可以引导形成只有一个晶胞厚度的沸石结构。沸石--微孔结晶铝硅酸盐--广泛应用于石油化学和精细化学合成1,2,3,因为其均匀微孔中的强酸位点可实现尺寸和形状选择性催化。但是,孔径小于 1 纳米的微孔的存在往往与扩散限制3,4,5 同时存在,从而对催化活性产生不利影响。这个问题可以通过减小沸石晶体的厚度来解决,这样可以减少扩散路径长度,从而改善分子扩散4,5。实现的方法有:合成沸石纳米晶体6;剥离层状沸石7,8,9;通过模板化策略10,11,12,13,14,15,16,17 或脱金属过程18,19,20,21,22 在微孔材料中引入中孔。但是,除了剥离法之外,这些方法都无法制备出厚度低于 5 纳米的 "超薄 "沸石。在这里,我们展示了适当设计的双功能表面活性剂可同时引导沸石结构在介孔和微孔长度尺度上的形成,从而产生厚度仅为 2 纳米的 MFI(ZSM-5,石化工业中最重要的催化剂之一)沸石纳米片,这相当于单个 MFI 单元尺寸的 b 轴尺寸。这些沸石外表面的大量酸性位点使它们在催化大分子有机物转化时具有很高的活性,而且晶体厚度的减少有利于扩散,从而大大抑制了甲醇到汽油转化过程中因焦炭沉积而导致的催化剂失活。我们希望我们的合成方法能应用于其他沸石,以提高它们在一系列重要催化应用中的性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


