The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 855
Abstract
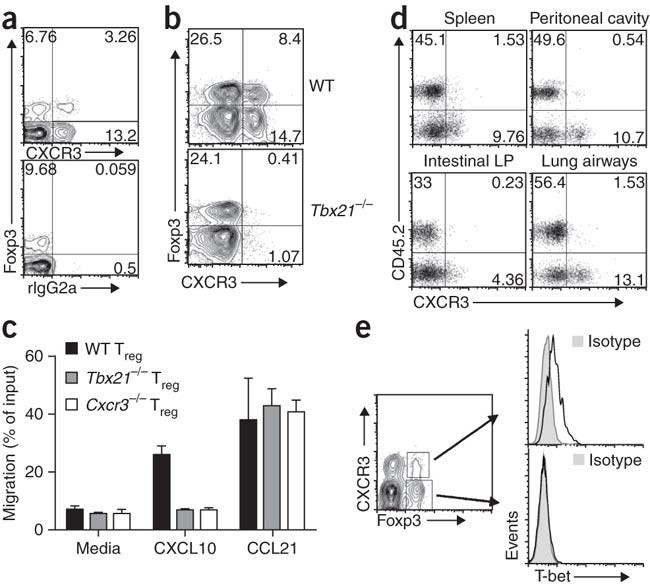
Several subsets of Foxp3+ regulatory T cells are known to exist. Campbell and colleagues show that one subset of regulatory T cells requires the transcription factor T-bet during T helper type 1–mediated immune responses in vivo. Several subsets of Foxp3+ regulatory T cells (Treg cells) work in concert to maintain immune homeostasis. However, the molecular bases underlying the phenotypic and functional diversity of Treg cells remain obscure. We show that in response to interferon-γ, Foxp3+ Treg cells upregulated the T helper type 1 (TH1)-specifying transcription factor T-bet. T-bet promoted expression of the chemokine receptor CXCR3 on Treg cells, and T-bet+ Treg cells accumulated at sites of TH1 cell–mediated inflammation. Furthermore, T-bet expression was required for the homeostasis and function of Treg cells during type 1 inflammation. Thus, in a subset of CD4+ T cells, the activities of the transcription factors Foxp3 and T-bet are overlaid, which results in Treg cells with unique homeostatic and migratory properties optimized for the suppression of TH1 responses in vivo.
转录因子 T-bet 控制着 1 型炎症期间调节性 T 细胞的稳态和功能
目前已知存在多个 Foxp3+ 调节性 T 细胞亚群。坎贝尔及其同事发现,在体内由 T 辅助细胞 1 型介导的免疫反应中,调节性 T 细胞的一个亚群需要转录因子 T-bet。Foxp3+ 调节性 T 细胞(Treg 细胞)的几个亚群协同维持免疫平衡。然而,Treg细胞的表型和功能多样性的分子基础仍然模糊不清。我们的研究表明,在对干扰素-γ做出反应时,Foxp3+ Treg细胞会上调T辅助细胞1型(TH1)指定转录因子T-bet。T-bet 促进了 Treg 细胞上趋化因子受体 CXCR3 的表达,T-bet+ Treg 细胞在 TH1 细胞介导的炎症部位聚集。此外,在 1 型炎症期间,Treg 细胞的平衡和功能需要 T-bet 的表达。因此,在 CD4+ T 细胞的一个亚群中,转录因子 Foxp3 和 T-bet 的活性相互叠加,从而产生了具有独特平衡和迁移特性的 Treg 细胞,这些特性最适于抑制体内的 TH1 反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


