Controlled Synthesis of α-CF2H or α-CF2Cl Styrenes from the Same Precursors: Dehydrazinative Hydrogenation or Chlorination of 3,3-Difluoroallyl Hydrazines
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
By carefully choosing the reaction conditions, we have developed the controllable FeCl3- or CuCl2-mediated dehydrazinative hydrogenation or chlorination of 3,3-difluoroallyl hydrazines to access α-CF2H or α-CF2Cl styrenes. The current reaction provides for the first time a facile method for the direct and selective synthesis of α-CF2H and α-CF2Cl styrenes starting from the same precursors, which is easy to scale up and displays a broad substrate scope and good functional group tolerance. Moreover, product derivatization experiments demonstrated that the resulting α-CF2Cl styrenes are practical and versatile building blocks for the diversified synthesis of fluorinated molecules.
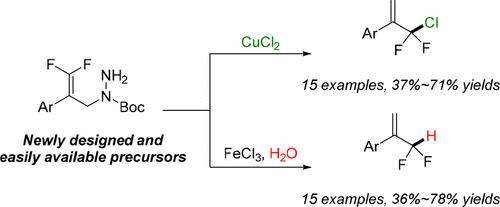
由相同前体控制合成α-CF2H或α-CF2Cl苯乙烯:3,3-二氟烯丙基肼的脱肼加氢或氯化
通过精心选择反应条件,我们开发了可控的FeCl3-或cucl2介导的3,3-二氟烯丙基肼脱氢或氯化反应,得到α-CF2H或α-CF2Cl苯乙烯。该反应首次为α-CF2H和α-CF2Cl苯乙烯的直接选择性合成提供了一种简便的方法,该方法易于扩大规模,具有底物范围广、官能团耐受性好等优点。此外,衍生化实验表明,所得α-CF2Cl苯乙烯是多种合成氟化分子的实用和通用的构建块。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


