Discovery of a first-in-class CDK2 selective degrader for AML differentiation therapy
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 52
Abstract
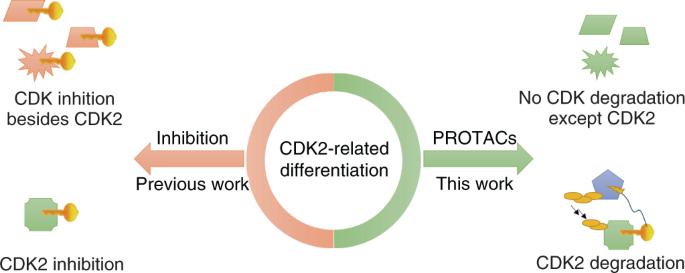
The discovery of effective therapeutic treatments for cancer via cell differentiation instead of antiproliferation remains a great challenge. Cyclin-dependent kinase 2 (CDK2) inactivation, which overcomes the differentiation arrest of acute myeloid leukemia (AML) cells, may be a promising method for AML treatment. However, there is no available selective CDK2 inhibitor. More importantly, the inhibition of only the enzymatic function of CDK2 would be insufficient to promote notable AML differentiation. To further validate the role and druggability of CDK2 involved in AML differentiation, a suitable chemical tool is needed. Therefore, we developed first-in-class CDK2-targeted proteolysis-targeting chimeras (PROTACs), which promoted rapid and potent CDK2 degradation in different cell lines without comparable degradation of other targets, and induced remarkable differentiation of AML cell lines and primary patient cells. These data clearly demonstrated the practicality and importance of PROTACs as alternative tools for verifying CDK2 protein functions. A selective and potent CDK2 degrader was developed that induced differentiation of acute myeloid leukemia cell lines and primary patient cells.
发现用于急性髓细胞白血病分化治疗的一流 CDK2 选择性降解剂
通过细胞分化而不是抗增殖来发现有效的癌症治疗方法仍然是一个巨大的挑战。使细胞周期蛋白依赖性激酶 2(CDK2)失活可克服急性髓性白血病(AML)细胞的分化停滞,这可能是治疗 AML 的一种有前途的方法。然而,目前还没有可用的选择性 CDK2 抑制剂。更重要的是,仅抑制 CDK2 的酶功能不足以促进明显的 AML 分化。为了进一步验证 CDK2 在急性髓细胞白血病分化中的作用和可药用性,需要一种合适的化学工具。因此,我们开发了第一类 CDK2 靶向蛋白水解靶向嵌合体(PROTACs),这种嵌合体能在不同细胞系中促进 CDK2 的快速、强效降解,而不会导致其他靶点的类似降解,并能诱导 AML 细胞系和原代患者细胞的显著分化。这些数据清楚地证明了PROTACs作为验证CDK2蛋白功能的替代工具的实用性和重要性。我们开发出了一种选择性强效 CDK2 降解剂,它能诱导急性髓性白血病细胞系和原代患者细胞分化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


