Cyanophycin and its biosynthesis: not hot but very cool
IF 12.7
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Covering: 1878 to early 2023Cyanophycin is a biopolymer consisting of a poly-aspartate backbone with arginines linked to each Asp sidechain through isopeptide bonds. Cyanophycin is made by cyanophycin synthetase 1 or 2 through ATP-dependent polymerization of Asp and Arg, or β-Asp-Arg, respectively. It is degraded into dipeptides by exo-cyanophycinases, and these dipeptides are hydrolyzed into free amino acids by general or dedicated isodipeptidase enzymes. When synthesized, chains of cyanophycin coalesce into large, inert, membrane-less granules. Although discovered in cyanobacteria, cyanophycin is made by species throughout the bacterial kingdom, and cyanophycin metabolism provides advantages for toxic bloom forming algae and some human pathogens. Some bacteria have developed dedicated schemes for cyanophycin accumulation and use, which include fine temporal and spatial regulation. Cyanophycin has also been heterologously produced in a variety of host organisms to a remarkable level, over 50% of the host's dry mass, and has potential for a variety of green industrial applications. In this review, we summarize the progression of cyanophycin research, with an emphasis on recent structural studies of enzymes in the cyanophycin biosynthetic pathway. These include several unexpected revelations that show cyanophycin synthetase to be a very cool, multi-functional macromolecular machine.
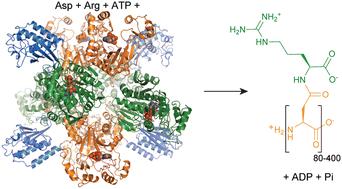
蓝藻素及其生物合成:不热但很冷
覆盖时间:1878年至2023年初蓝藻素是一种生物聚合物,由多天冬氨酸骨架和精氨酸通过异肽键连接到每个Asp侧链上。藻青素是由藻青素合成酶1或2分别通过Asp和Arg或β-Asp-Arg的atp依赖性聚合而产生的。它被外蓝藻酶降解成二肽,这些二肽被一般或专用的异二肽酶水解成游离氨基酸。当合成时,蓝藻素链聚合成大的,惰性的,无膜的颗粒。虽然在蓝藻中发现,但整个细菌王国的物种都能产生蓝藻素,并且蓝藻素的代谢为形成有毒水华的藻类和一些人类病原体提供了优势。一些细菌已经形成了专门的蓝藻素积累和利用方案,包括精细的时间和空间调控。蓝藻素也在多种寄主生物中异种产生,达到显著水平,占寄主干质量的50%以上,具有多种绿色工业应用潜力。本文综述了藻青素的研究进展,重点介绍了藻青素生物合成途径中酶的结构研究进展。其中包括一些意想不到的启示,显示蓝藻素合成酶是一个非常酷,多功能的大分子机器。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Central Science
Chemical Engineering-General Chemical Engineering
CiteScore
25.50
自引率
0.50%
发文量
194
审稿时长
10 weeks
期刊介绍:
ACS Central Science publishes significant primary reports on research in chemistry and allied fields where chemical approaches are pivotal. As the first fully open-access journal by the American Chemical Society, it covers compelling and important contributions to the broad chemistry and scientific community. "Central science," a term popularized nearly 40 years ago, emphasizes chemistry's central role in connecting physical and life sciences, and fundamental sciences with applied disciplines like medicine and engineering. The journal focuses on exceptional quality articles, addressing advances in fundamental chemistry and interdisciplinary research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


