Interaction mechanism of cholesterol/β-cyclodextrin complexation by combined experimental and computational approaches
Abstract
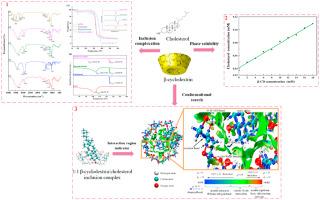
It is crucial to accurately describe non-covalent weak interactions between the components of cholesterol/β-cyclodextrin inclusion complex in the modulation of its stability. Experimental and theoretical infrared spectra confirmed that the inclusion complex was successfully prepared. Phase solubility studies suggested the formation of 1:1 stoichiometric inclusion complex of cholesterol with β-CD. The inclusion complex showed higher decomposed temperature (327.3 °C) than physical mixture (321.6 °C), indicating improved thermal stability of inclusion complex. Conformational search and density functional theory calculations were performed to find the most stable conformation with the lowest complexation energy (−129.66 kJ/mol). Dispersion energy from van der Waals (−283.31 kJ/mol) contributed greatly on total bonding energy (−204.72 kJ/mol). The bond lengths of hydrogen bonds (O127⋅⋅⋅H221 and O220⋅⋅⋅H107) were 1.77 and 1.62 Å, respectively. Van der Waals and hydrogen bonds were necessary to drive spontaneous formation and maintain the stability of inclusion complex. This study revealed a deep insight into the formation mechanism of cholesterol/β-CD inclusion complex using computational and experimental approaches, providing theoretical basis for the removal of cholesterol from hydrocolloid-containing systems.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


