An extracellular activator of the Drosophila JAK/STAT pathway is a sex-determination signal element
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 81
Abstract
Metazoans use diverse and rapidly evolving mechanisms to determine sex. In Drosophila melanogaster an X-chromosome-counting mechanism determines the sex of an individual by regulating the master switch gene, Sex-lethal (Sxl)1. The X-chromosome dose is communicated to Sxl by a set of X-linked signal elements (XSEs), which activate transcription of Sxl through its ‘establishment’ promoter, SxlPe. Here we describe a new XSE called sisterlessC (sisC) whose mode of action differs from that of previously characterized XSEs, all of which encode transcription factors that activate Sxl Pe directly. In contrast, sisC encodes a secreted ligand for the Drosophila Janus kinase (JAK) and ‘signal transducer and activator of transcription’ (STAT) signal transduction pathway and is allelic to outstretched (os, also called unpaired). We conclude that sisC works indirectly on Sxl through this signalling pathway because mutations in sisC or in the genes encoding Drosophila JAK or STAT reduce expression of SxlPe similarly. The involvement of os in sex determination confirms that secreted ligands can function in cell-autonomous processes. Unlike sex signals for other organisms, sisC has acquired its sex-specific function while maintaining non-sex-specific roles in development, a characteristic that it shares with all other Drosophila XSEs2.
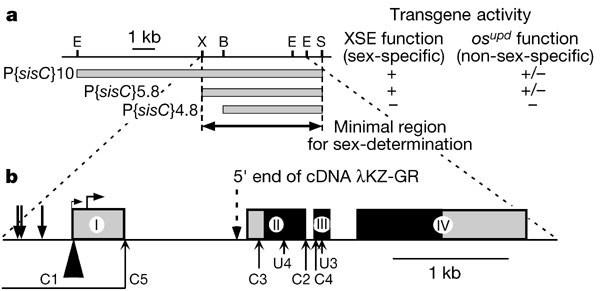
果蝇 JAK/STAT 通路的细胞外激活剂是性别决定信号元件
后生动物使用多种快速进化的机制来决定性别。在黑腹果蝇中,X 染色体计数机制通过调节主开关基因--性致死基因(Sxl)1 来决定个体的性别。X 染色体剂量通过一组 X 连锁信号元件(XSE)传递给 Sxl,这些信号元件通过其 "建立 "启动子 SxlPe 激活 Sxl 的转录。在这里,我们描述了一种名为sisterlessC(sisC)的新的XSE,它的作用模式不同于之前表征的XSE,所有这些XSE都编码直接激活Sxl Pe的转录因子。与此相反,sisC 编码果蝇 Janus 激酶(JAK)和 "转录信号转导和激活因子"(STAT)信号转导途径的分泌配体,并且与外伸体(os,也称未配对)等位。我们的结论是,sisC 通过这一信号转导途径间接作用于 Sxl,因为 sisC 或编码果蝇 JAK 或 STAT 的基因发生突变,同样会降低 SxlPe 的表达。os参与性别决定证实了分泌配体可在细胞自主过程中发挥作用。与其他生物的性别信号不同,sisC 在发育过程中保持非性别特异性作用的同时获得了性别特异性功能,这是它与果蝇所有其他 XSEs2 的共同特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


