Practical, Catalytic Enantioselective Hydrogenation to Synthesize N-Unprotected β-Amino Esters
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 27
Abstract
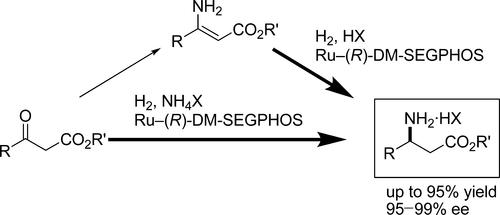
Practical and simple catalytic enantioselective hydrogenation reactions to synthesize N-unprotected β-amino esters have been developed: (1) asymmetric hydrogenation of N-unprotected β-enamine ester and (2) asymmetric direct reductive amination of β-keto esters using ammonium salts. A Ru–DM-SEGPHOS complex was used as the catalyst in both cases and gave high enantioselectivity, high reactivity, and wide substrate applicability. These protocols greatly reduced reaction time and waste compared to conventional synthetic routes. The direct reductive amination route was demonstrated on a >100 kg scale.
实用的催化对映选择性加氢合成n -无保护β-氨基酯
开发了实用简单的催化对映选择性加氢合成n -无保护β-氨基酯的反应:(1)n -无保护β-烯胺酯的不对称加氢反应和(2)用铵盐直接还原β-酮酯的不对称氨化反应。在这两种情况下,Ru-DM-SEGPHOS配合物作为催化剂,具有高对映选择性,高反应活性和广泛的底物适用性。与传统的合成路线相比,这些方案大大减少了反应时间和浪费。在100公斤的规模上演示了直接还原胺化路线。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


