A simple and highly effective oxidative chlorination protocol for the preparation of arenesulfonyl chlorides
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 27
Abstract
2,4-Dichloro-5,5-dimethylhydantoin (DCDMH) was found to be a mild and efficient reagent for the direct oxidative conversion of sulfur compounds to the corresponding arenesulfonyl chlorides in good to excellent yields through oxidative chlorination. The method is suitable for many types of sulfur substrates (thiols, disulfides, and benzylic sulfides). The overall process is simple, practical, and it provides convenient access to a variety of aryl or heteroarylsulfonyl chlorides. The mild reaction conditions and the broad substrate scope render this method attractive and complementary to existing syntheses of aryl or heteroarylsulfonyl chlorides.
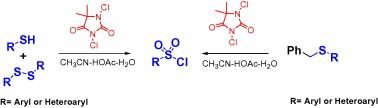
一种简单高效的氧化氯化法制备芳烃磺酰氯
2,4-二氯-5,5-二甲基海因(DCDMH)是一种温和、高效的试剂,可通过氧化氯化反应将含硫化合物直接氧化转化为相应的芳烃磺酰氯。该方法适用于多种类型的含硫底物(硫醇、二硫化物和苯基硫化物)。整个过程简单、实用,并且可以方便地获得各种芳基或杂芳基磺酰氯。温和的反应条件和广泛的底物范围使该方法具有吸引力,并与现有的芳基或杂芳基磺酰氯的合成相补充。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


